- Histidine kinase
protein
Name = Histidine Kinase
width =
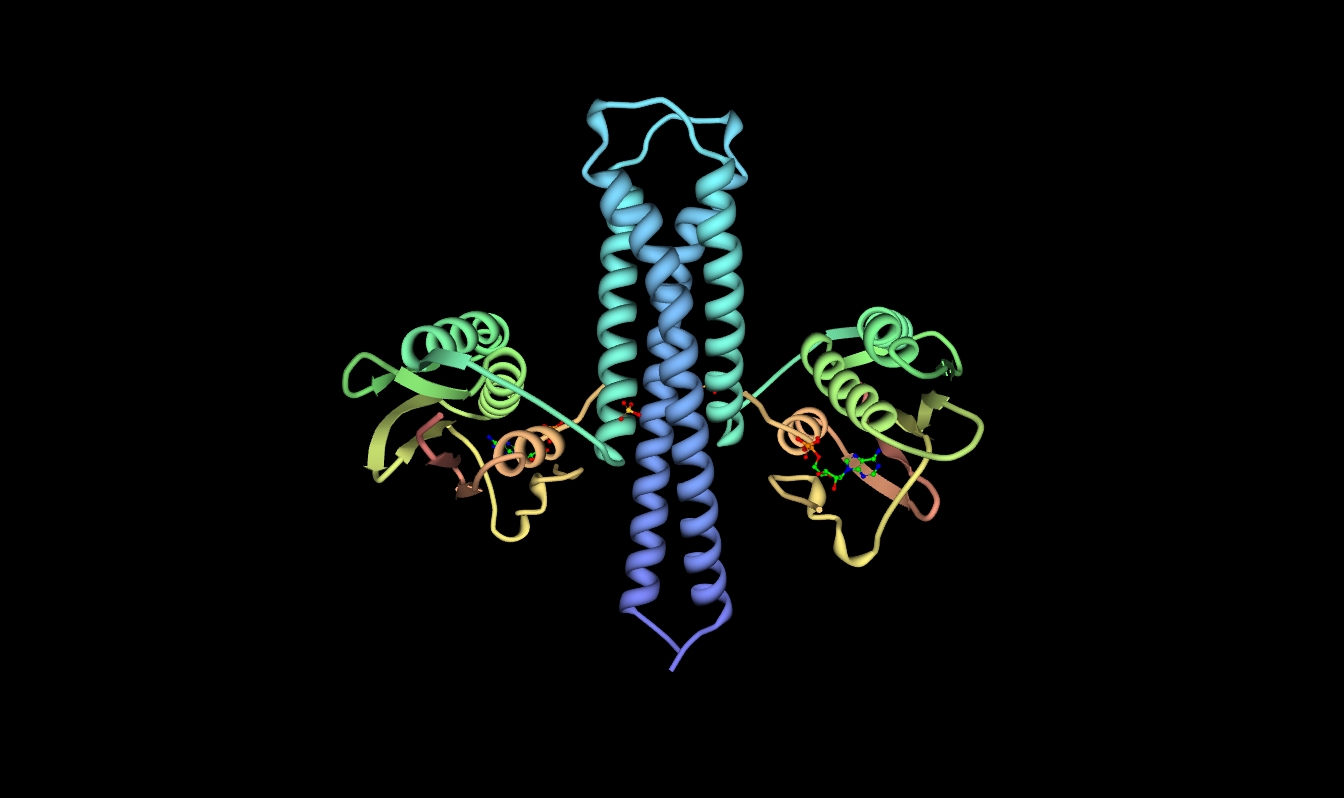
caption = Crystallographic structure of ATP:protein-L-histidine N-phosphotransferase based on the PDB|2c2a coordinates.
Symbol = HK
AltSymbols =
ATC_prefix=
ATC_suffix=
ATC_supplemental=
CAS_number= 99283-67-7
CAS_supplemental=
DrugBank=
EntrezGene =
HGNCid =
OMIM =
PDB =2C2A
RefSeq =
UniProt =
ECnumber = 2.7.13.3
Chromosome =
Arm =
Band =
LocusSupplementaryData = Inenzymology , a histidine kinase (EC number|2.7.13.3) is anenzyme that catalyzes thechemical reaction :ATP + protein L-histidine ADP + protein N-phospho-L-histidine.Thus, the two substrates of this enzyme are ATP and
protein L-histidine , whereas its two products are ADP andprotein N-phospho-L-histidine . Histidine Kinases (HK) are multifunctional, typically transmembrane, enzymes of thetransferase class that serve a major role in signal transduction through the cellular membrane. The vast majority of HKs are homodimers and exhibitautokinase , phosphotransfer, and phosphatase activity. The mechanisms for these reactions have not been completely elucidated, but current evidence suggests that the catalytic domain of one dimeric unit may rotate in such a way that the ATP binding pocket of that unit can come into contact with a particular histidine residue on the opposite unit and a nucleophilic addition results in a phosphorylated histidine (Marina et al., 2005).Other names in common use include EnvZ, histidine kinase (ambiguous), HK1, HP165, and Sln1p. This enzyme participates in 4 metabolic pathways: two-component system - general,
bacterial chemotaxis - general ,bacterial chemotaxis - organism-specific , andtype ii secretion system .tructure and Function
HKs are comprised of several domains starting with a short N-terminal cytoplasmic portion connected to an extracellular sensing domain via a transmembrane α helix. A second transmembrane α helix connects the extracellular domain to the C-terminal cytoplasmic catalytic domain. HKs are known to serve roles in many different signal transduction pathways, so it is not surprising that the extracellular sensing domain is not very well conserved in the HK family. In contrast, the cytoplasmic domain tends to have high sequence homology and contains several well-known motifs. These motifs include the H, N, G1, F, and G2 boxes (Parkinson and Kofoid, 1992). The autophosphorylation H-box is contained in the N-terminal dimerization and histidine phosphotransfer (DHp) domain. In HK853-CD, crystallized from Thermotoga maritima, this domain is a helical-hairpin and is formed by residues 232-317. The histidine phosphorylation site is located at His260. The N, G1, F and G2 boxes are contained in the C-terminal catalytic and ATP-binding (CA) domain. This domain is formed by residues 323-489 and forms a structure known as an α/β sandwich fold. This particular fold has one layer composed of a 5-stranded β sheet and the other layer is made of three α helices. The dimeric unit is held together by a four-helix bundle, formed when the C-terminal segments of the α1 helices on each subunit interact in an antiparallel manner with both α2 helices. The stability of the dimer is aided by several interactions at the interface between the DHps of each monomer. These include hydrophobic interactions between conserved hydrophobic residues as well as two hydrogen bonds (Thr252-Glu316’ and Arg263-Asn307’) and one
salt bridge (Lys270-Glu303’). Further interactions are mediated via hydrogen bonds to water within a cavity inside the coiled coil and flanked by hydrophobic residues.AMPPNP in the crystal structure. During crystallization, the analog was hydrolyzed into a product similar to ADP.
The final side of the ATP binding pocket is conveniently named the “ATP lid.” The stability of this structure is mediated by the presence of the γ phosphate and thus the Mg2+ ion in the binding site. Also the presence of the nucleotide base has proved to play a significant role in stabilization of the lid in a closed conformation. The ATP lid is connected via hydrophobic residues to the rest of the protein. The γ phosphate of ATP is somewhat exposed allowing for
dephosphorylation . Upon ATP binding in this pocket, it is believed that a conformational change occurs allowing the rotation of the CA domain to come into contact with the DHp of the other monomer and thus allowing the conserved His260 to rest near the γ phosphate. The Nε of His260 then attacks the γ phosphate of ATP in anucleophilic addition and bumps off ADP as its leaving group.Role in Fungal Infections
Research suggests that a two-component system, involving histidine kinase and a variable response regulator protein, may be critical to the virulence of some fungal strains such as
Candida albicans , which is often responsible for causingcandidiasis in immunocompromised persons (Torosantucci et al. 2002). C. albicans with a deletion of CHK1, the two-component histidine kinase gene, show defects inmorphogenesis and a drastic decrease in the cell’s ability to resist elimination by human neutrophils. As humans lack this two component system, it may be a good target for anti-microbial agents in order to treat candidiasis.tructural studies
As of late 2007, 9 structures have been solved for this class of enzymes, with PDB accession codes PDB link|1P0Z, PDB link|2CMN, PDB link|2GJ3, PDB link|2HJE, PDB link|2J48, PDB link|2O9B, PDB link|2O9C, PDB link|2R78, and PDB link|2R8R.
References
*
*
*
*
*
*
*
*
*
*
*
*External links
Gene Ontology (GO) codes
Wikimedia Foundation. 2010.
