- Monellin
-
Monellin Chain A and B 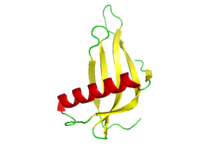
X-ray crystal structure of a single-chain monellin protein.[1] Identifiers Symbol MNEI PDB 2O9U UniProt P02882 Other data Monellin is a sweet protein which was discovered in 1969 in the fruit of the West African shrub known as serendipity berry (Dioscoreophyllum cumminsii), it was first reported as a carbohydrate.[2] The protein was named in 1972 after the Monell Chemical Senses Center in Philadelphia, U.S.A., where it was isolated and characterized.[3]
Contents
Protein composition
So far, five high intensity sweet proteins have been reported: monellin (1969), thaumatin (1972), pentadin (1989), mabinlin (1983) and brazzein (1994).[4]
Monellin's molecular weight is 10.7 kDa. Monellin is a two noncovalently associated polypeptide chains: an A chain sequence with 44 amino acid residues, and a B chain with 50 residues.[3][5]Monellin chain A (44 AA):
REIKGYEYQL YVYASDKLFR ADISEDYKTR GRKLLRFNGP VPPP
Monellin chain B (50 AA):
GEWEIIDIGP FTQNLGKFAV DEENKIGQYG RLTFNKVIRP CMKKTIYEEN
Amino acid sequence of the sweet protein monellin adapted from Swiss-Prot biological database of protein.[6][7]Monellin has a secondary structure consisting of five Beta-strands that form an anti-parallel Beta-sheet and a 17-residue Alpha-helix.[1]
In its natural form, monellin is composed of 2 chains (A and B, of 44 and 50 amino acids as shown above), but is unstable at high temperatures or at extremes of pH.[1] In order to enhance its stability, single-chain monellin proteins were created in which the two natural chains are joined via a Gly-Phe dipeptide linker.[1] This modified version of the protein (MNEI) has been studied using NMR and X-ray diffraction.
The image displayed on this page shows the structure of monellin derived from X-ray diffraction at a 1.15 ångström resolution.[1] The high resolution used to obtain the structure provided a more detailed look at monellin and its properties than previously possible.[1]
In addition to its secondary structure, four stably bound sulfate ions were located on the monellin protein, three on the concave face of the protein and one on the convex face of the protein.[1] The sulfate ion on the convex face of the protein is of particular interest because it lies adjacent to a patch of positive surface potential, which may be important in electrostatic interactions with the negative T1R2-T1R3 sweet taste protein receptor.[1]
Sweetness properties
Monellin is perceived as sweet by humans and some Old World primates, but is not preferred by other mammals.[1] The relative sweetness of monellin varies from 800 to 2000 times sweeter than sucrose, depending on the sweet reference it is assessed against. It is reported to be 1500-2000 times sweeter than a 7% sucrose solution on a weight basis<[8][9] [10] and 800 times sweeter than sucrose when compared with a 5% sucrose solution on a weight basis.[11] Monellin has a slow onset of sweetness and lingering aftertaste. Like miraculin, monellin's sweetness is pH dependent; the protein is tasteless below pH 2 and above pH 9. Blending the sweet protein with bulk and/or intense sweeteners reduces the persistent sweetness and shows a synergistic sweet effect.[12]
Heat over 50°C at low pH denatures monellin proteins causing a loss of the sweetness.[12]As a sweetener
Monellin can be useful for sweetening some foods and drinks as it is a protein readily soluble in water due to its hydrophilic properties. However it may have limited application because it denatures under high temperature conditions which makes it unsuitable for processed food. It may be relevant as noncarbohydrate tabletop sweetener,especially for individuals such as diabetics who must control their sugar intake.[1]
In addition, monellin is costly to extract from the fruit and the plant is difficult to grow. Alternative production such as chemical synthesis and expression in micro-organisms are being investigated. For instance, monellin has been expressed successfully in yeast (Candida utilis)[13] and synthesised by solid-phase method.[14] The synthetic monellin produce by yeast was found to be 4000 times sweeter than sucrose when compared to 0.6% sugar solution.
Legal issues are the main barrier in widespread use as a sweetener as Monellin has no legal status in the European Union or the United States. However, it is approved in Japan as a harmless additive, according to the List of Existing Food Additives issued by the Ministry of Health and Welfare (published in English by JETRO).See also
References
- ^ a b c d e f g h i j PDB 2O9U; Hobbs JR, Munger SD, Conn GL (March 2007). "Monellin (MNEI) at 1.15 A resolution". Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 63 (Pt 3): 162–7. doi:10.1107/S1744309107005271. PMC 2330190. PMID 17329805. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2330190.
- ^ GE Inglett, JF May. Serendipity berries - Source of a new intense sweetener. J Food Sci 1969, 34:408-411.
- ^ a b Morris JA, Martenson R, Deibler G, Cagan RH (January 1973). "Characterization of monellin, a protein that tastes sweet". J. Biol. Chem. 248 (2): 534–9. PMID 4684691. http://www.jbc.org/cgi/content/abstract/248/2/534.
- ^ Biopolymers. Volume 8. Polyamides and Complex Proteinaceous Materials II. Sweet-tasting Proteins. I Faus and H Sisniega. p203-209. 2004. Eds. Wiley-VCH. ISBN 3-527-30223-9.
- ^ Ogata C, Hatada M, Tomlinson G, Shin WC, Kim SH (1987). "Crystal structure of the intensely sweet protein monellin". Nature 328 (6132): 739–42. doi:10.1038/328739a0. PMID 3614382.
- ^ UniProtKB/Swiss-Prot database entry #P02881
- ^ UniProtKB/Swiss-Prot database entry #P02882
- ^ Kim NC, Kinghorn AD (December 2002). "Highly sweet compounds of plant origin". Arch. Pharm. Res. 25 (6): 725–46. doi:10.1007/BF02976987. PMID 12510821.
- ^ Gelardi, Robert C.; Nabors, Lyn O'Brien (1991). Alternative sweeteners. New York: M. Dekker. ISBN 0-8247-8475-8.
- ^ AD kinghorn and CM Compadre. Less common high-potency sweeteners. In Alernative Sweeteners: Second Edition, Revised and Expanded, L O'Brien Nabors,Ed., New York, 1991. ISBN 0-8247-8475-8.
- ^ US patent 4122205, Burge MLE, Nechutny Z, "Sweetening compositions containing protein sweeteners", issued 1978-10-24
- ^ a b Nabors, Lyn O'Brien; Lyn O'Brien-Nabors (2001). Alternative sweeteners / edited by Lyn O'Brien Nabors. New York, N.Y: Marcel Dekker. ISBN 0-8247-0437-1.
- ^ XL Zhang, T Ito, K Kondo, T Kobayashi and H Honda. Production of single chain recombinant monellin by high cell density culture of genetically engineered Candida utilis using limited feeding of sodium ions. Journal of Chemical Engineering of Japan 2002. 35: 654-659.
- ^ M Kohmura, T Mizukoshi, N Nio, EI Suzuki and Y Ariyoshi. Structure–taste relationships of the sweet protein monellin. Pure Appl. Chem., Vol. 74, 1235-1242, 2002.
Categories:- Sweeteners
- Proteins
Wikimedia Foundation. 2010.
