- Darunavir
-
Darunavir 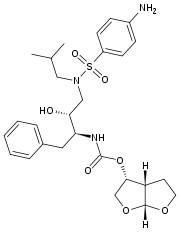
Systematic (IUPAC) name [(1R,5S,6R)-2,8-dioxabicyclo[3.3.0]oct-6-yl] N-[(2S,3R)-4- [(4-aminophenyl)sulfonyl- (2-methylpropyl)amino]-3-hydroxy-1-phenyl- butan-2-yl] carbamate Clinical data Trade names Prezista AHFS/Drugs.com monograph MedlinePlus a607042 Pregnancy cat. B Legal status ? Routes oral Pharmacokinetic data Protein binding 95% Metabolism hepatic (CYP) Half-life 15 hours Identifiers CAS number 206361-99-1 
ATC code J05AE10 PubChem CID 213039 DrugBank DB01264 ChemSpider 184733 
UNII YO603Y8113 
KEGG D03656 
ChEBI CHEBI:367163 
ChEMBL CHEMBL1323 
Chemical data Formula C27H37N3O7S Mol. mass 547.665 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Darunavir (brand name Prezista, formerly known as TMC114) is a drug used to treat HIV infection. It is in the protease inhibitor class. Prezista is an OARAC recommended treatment option for treatment-naïve and treatment-experienced adults and adolescents[1]. Developed by pharmaceutical company Tibotec, darunavir is named after Arun K. Ghosh, the chemist who discovered the molecule at the University of Illinois at Chicago. It was approved by the Food and Drug Administration (FDA) on June 23, 2006.[2]
Darunavir is a second-generation protease inhibitor (PIs), designed specifically to overcome problems with the older agents in this class, such as indinavir. Early PIs often have severe side effects and drug toxicities, require a high therapeutic dose, are costly to manufacture, and show a disturbing susceptibility to drug resistant mutations. Such mutations can develop in as little as a year of use, and effectively render the drugs useless.
Darunavir was designed to form robust interactions with the protease enzyme from many strains of HIV, including strains from treatment-experienced patients with multiple resistance mutations to PIs.[3][4]
Darunavir received much attention at the time of its release, as it represents an important treatment option for patients with drug-resistant HIV. Patient advocacy groups pressured developer Tibotec not to follow the previous trend of releasing new drugs at prices higher than existing drugs in the same class. Darunavir was priced to match other common PIs already in use, such as the fixed-dose combination drug lopinavir/ritonavir. The drug costs around $9,000 for a one year supply.[5][6][7]
Contents
Efficacy
Prezista is an OARAC (DHHS) recommended treatment option for treatment-naïve and treatment-experienced adults and adolescents[1].
Darunavir showed comparable efficacy to lopinavir/ritonavir at 96 weeks with a once-daily dosing in treatment-naïve patients[8]. It was approved by the FDA for treatment-naive patients on October the 21st 2008.[9]
Darunavir showed superiority to lopinavir/ritonavir and other protease inhibitors in the POWER trials. The POWER 1 and POWER 2 were designed for treatment-experienced patients, together with supportive data from the POWER 3 analysis. [10] The patients eligible for these studies had experience with at least one protease inhibitor, one non-nucleoside reverse transcriptase inhibitor (NNRTI) and two nucleoside reverse transcriptase inhibitors (NRTI), and had one or more primary protease inhibitor mutations.
Darunavir also showed superior results to lopinavir in the TITAN trials (pre-planned, secondary endpoint, week 48), which was designed for patients with less advanced HIV disease compared to the POWER trials.[11]
ARTEMIS trial
ARTEMIS includes 689 treatment-naive participants with a baseline viral load of at least 5000 copies/mL who were randomly assigned to receive 800/100 mg once-daily darunavir/ritonavir or 800/200 mg lopinavir/ritonavir given once- or twice-daily. At 96 weeks, darunavir/ritonavir remained non-inferior to lopinavir/ritonavir.
- In an intent-to-treat analysis, significantly more patients in the darunavir/ritonavir arm achieved HIV RNA below 50 copies/mL compared with the lopinavir/ritonavir arm (79% vs. 71%; p = 0.012).
- Response rates in the darunavir/ritonavir arm were statistically superior to those in the lopinavir/ritonavir arm for patients with high baseline viral load and low baseline CD4 count.
- Among patients with baseline viral load below 100,000 copies/mL, 76% of patients in the darunavir/ritonavir arm and 63% in the lopinavir/ritonavir arm achieved HIV RNA below 50 copies/mL (p = 0.023).
- Once-daily darunavir/ritonavir was generally safe and well tolerated.
- Fewer patients in the darunavir/ritonavir arm discontinued treatment due to adverse events (4% vs. 9%).
- Patients taking darunavir/ritonavir were less likely to have moderate to severe (grade 2-4) treatment-related diarrhea (4% vs. 11%; p < 0.001).
- Grade 2-4 treatment-related rash occurred infrequently in both arms (3% with darunavir/ritonavir vs. 1% with lopinavir/ritonavir; p = 0.273).
- Patients taking darunavir/ritonavir had smaller average increases in triglycerides (0.1 vs. 0.8 mmol/L, or 12% vs. 50%) and total cholesterol (0.6 vs. 0.9 mmol/L, or 15% vs. 23%) (both p < 0.0001).
TITAN trial
Analysis of 595 treatment-experienced patients being lopinavir/r-naïve, HIV-1 infected adults with a viral load of greater than 1000 HIV-1 RNA copies/mL. Pre-planned secondary endpoint findings include:
- 71 percent of patients in the darunavir/r arm reached an undetectable viral load (less than 50 copies/mL) vs. 60 percent of patients in the lopinavir/r arm, a statistically significant difference (p = 0.005)
- 77 percent of patients in the darunavir/r arm achieved at least a 1 log10 reduction in HIV RNA vs. 69 percent in the lopinavir/r arm, a statistically significant difference (p = 0.028)
- The median increase from baseline in CD4 cell count was similar between the darunavir/r and lopinavir/r arms (88 cells per cubic millimeter vs. 81 cells per cubic millimeter)
Development of resistance also was studied. Findings include:
- 10 percent of patients in the darunavir/r arm experienced virological failure vs. 22 percent of patients in the lopinavir/r arm
- Among patients experiencing virologic failure who had baseline and endpoint genotype data, 21 percent of patients in the darunavir/r arm developed primary PI resistance mutations vs. 36 percent of patients in the lopinavir/r arm, and 14 percent of patients in the darunavir/r arm developed primary NRTI resistance mutations vs. 27 percent of patients in the lopinavir/r arm
POWER 1 and POWER 2 trials
A pooled analysis of results from POWER 1 and POWER 2 demonstrated that after 24 weeks:
- Significantly more treatment-experienced patients achieved a reduction in viral load at the 24-week primary endpoint with darunavir, compared with the investigator-selected PI (70% vs. 21%, respectively).
- Almost four times as many treatment-experienced patients (45%) have achieved an undetectable viral load with the darunavir containing regimen, compared with the investigator-selected PI arm (12%).
- In treatment-experienced patients, the darunavir containing regimen increases CD4 cell counts five times more than the investigator-selected PI arm (92 cells/mm3 vs. 17 cells/mm3, respectively) (Johnson & Johnson Press Release, 2006; Lazzarin, 2005)
The efficacy results of POWER 1 and POWER 2 are confirmed by data from a large, non-randomized, open-label analysis known as POWER 3. After 24 weeks:
- 65 percent of patients achieved a reduction in viral load of 1 log10 or more, versus baseline.
- 40 percent of patients reached undetectable virus levels (less than 50 HIV RNA copies/mL). (Molina, 2005)
Pharmacoeconomic considerations
In the US and UK, healthcare costs were estimated to be lower with boosted darunavir than with investigator-selected control protease inhibitors in treatment-experienced patients.[12]
Safety
As other antivirals, darunavir does not cure HIV infection or AIDS, and does not prevent passing HIV to others.
In studies, darunavir was generally well tolerated. Mild to moderate rash was seen in 7% of patients. Some patients developed severe rash. In clinical studies, 0.3% of patients discontinued due to rash. The most common moderate to severe side effects associated with darunavir include diarrhea (2.3%), headache (3.8%), abdominal pain (2.3%), constipation (2.3%), and vomiting (1.5%). Four percent of patients discontinued treatment due to adverse events. People who are allergic to darunavir or any of its ingredients, or ritonavir (Norvir) should not take darunavir.
There were few relevant drug-drug interactions with other medications commonly used in HIV patient populations, such as other antiretroviral medications, proton pump inhibitors, and H2 receptor antagonists. St. John's Wort may reduce its effectiveness by interaction with CYP3A. Patients should talk to their healthcare provider about all the medicines they are taking or plan to take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Before taking darunavir, patients should tell their healthcare provider if they have any medical conditions, including diabetes, liver problems, hemophilia, or allergy to sulfa medicines and should tell their doctor if they are pregnant or planning to become pregnant, or are nursing. Darunavir should be used with caution in patients with hepatic impairment.
High blood sugar, diabetes or worsening of diabetes, muscle pain, tenderness or weakness, and increased bleeding in people with hemophilia have been reported in patients taking protease inhibitor medicines like darunavir. Changes in body fat have been seen in some patients taking anti-HIV medicines, including loss of fat from legs, arms and face, increased fat in the abdomen and other internal organs, breast enlargement and fatty lumps on the back of the neck. The cause and long-term health effects of these conditions are not known at this time.
Clinical laboratory safety observed in the darunavir group was comparable to the control group. (Product Monograph, Darunavir)
Dosing and administration
The recommended oral dose of darunavir tablets is 600 mg (two 300 mg tablets) twice daily (BID) taken with ritonavir 100 mg BID and with food. The drug can be taken with any type of food.
Additional Studies Involving Darunavir
- TMC114-C211: Investigating a dose of 800 mg of the drug boosted with 100 mg of ritonavir once daily in treatment-naïve patients.
- TMC114-C214: Investigating a dose of 600 mg of the drug boosted with 100 mg of ritonavir twice daily in moderately treatment-experienced patients.
- DUET trial: The drug is being studied with TMC125, an investigational non-nucleoside reverse transcriptase inhibitor, in one of the few HIV clinical trials to involve two investigational HIV treatments in treatment-experienced patients. (Tibotec 2006)
See also
Notes
- ^ a b Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents, November 3, 2008, Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC). full guidelines.
- ^ Rodger D MacArthura, Darunavir: promising initial results, doi:10.1016/S0140-6736(07)60499-1
- ^ Ghosh AK, Dawson ZL, Mitsuya H (2007). "Darunavir, a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV". Bioorg. Med. Chem. 15 (24): 7576–80. doi:10.1016/j.bmc.2007.09.010. PMC 2112938. PMID 17900913. http://linkinghub.elsevier.com/retrieve/pii/S0968-0896(07)00783-3. Retrieved 2007-12-22.
- ^ Darunavir-ritonavir more effective than Lopinavir-ritonavir in HIV infected, treatment-experienced patients, The Lancet, 2007, 370, article URL
- ^ Liz Highleyman, Patient Advocates Commend Pricing of New PI Darunavir, HIV and HCV news
- ^ Darunavir - first molecule to treat drug-resistant HIV, Medical news
- ^ Retaining Efficacy Against Evasive HIV, Chemical and engineering news
- ^ hivandhepatitis.com, Efficacy and Safety of Boosted Darunavir (Prezista) Are Superior to Lopinavir/ritonavir (Kaletra) at 96 Weeks: ARTEMIS Trial, 2008-10-28, URL.
- ^ hivandhepatitis.com, Darunavir (Prezista) Receives Full Traditional Approval, Dose Set for Treatment-naive Patients, Caution Urged for Pregnant Women, 2008-10-24, URL.
- ^ Bonaventura Clotet, Nicholas Bellos, Jean-Michel Molina, David Cooper, Jean-Christophe Goffard, Adriano Lazzarin, Andrej Wöhrmann, Christine Katlama, Timothy Wilkin, Richard Haubrich, et al., Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials, The Lancet, Volume 369, Issue 9568, 7 April 2007-13 April 2007, Pages 1169-1178.
- ^ José Valdez Madruga, Daniel Berger, Marilyn McMurchie, Fredy Suter, Denes Banhegyi, Kiat Ruxrungtham, Dorece Norris, Eric Lefebvre, Marie-Pierre de Béthune, Frank Tomaka, et al., Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial, Pages 49-58. DOI 10.1016/S0140-6736(07)61049-6
References
- Lazzarin A, Queiroz-Telles F, Frank I, Rockstroh J, Walmsley S, De Paepe E, Vangeneugden T, Spinosa-Guzman S and Lefebvre E Lazzarin A, et al. XVI IAC 2006.
- Johnson & Johnson FDA Approval Press Release, June 23 2006, http://www.jnj.com/news/jnj_news/20060623_191250.htm;jsessionid=NT1BC4RC4RHKYCQPCAOWU3YKB2IIWTT
- Molina JM, Cohen C, Katlama C et al. TMC114/r in treatment-experienced HIV patients in power 3: 24-week efficacy and safety analysis. Poster abstract TUPE0060.
- Janssen-Ortho, Darunavir Mongraph information. Updated 2006. http://www.janssen-ortho.com/JOI/pdf_files/Darunavir_E.pdf
- TMC114, Tibotec, http://www.tibotec.com/bgdisplay.jhtml?itemname=HIV_tmc114
- Ghosh, A. K., et al. Bioorg. Med. Chem. Lett. 1998, 8, 687-90;
- Mitsuya, H. Ghosh, A. K., et al. J. Virology 2002, 76, 1349;
- Ghosh, A. K. Duzguiness, N., et al. Antiviral Res. 2002, 54, 29;
- Koh, Y., Ghosh, A. K., Mitsuya, H., et al. Antimicrobial Agents and Chemotherapy, 2003, 47, 3123
- Ghosh, A. K., Mitsuya, H., et al. ChemMedChem 2006, 1, 937
- A. K. Ghosh, B. D. Chapsal, I. T. Weber, H. Mitsuya. Acc. Chem. Res. 2007, ASAP.
- A. K. Ghosh, Z. L. Dawson, H. Mitsuya. Bioorg. Med. Chem. 2007, 15, 7576.
External links
- Darunavir FAQ at AIDSmeds.com
- Drug information in PDF
- Arun Ghosh group
- Tibotec
Categories:- Protease inhibitors
- Sulfonamides
- Carbamates
Wikimedia Foundation. 2010.
