- Vanadium(III) chloride
-
Vanadium(III) chloride 

 Vanadium(III) chloride
Vanadium(III) chloride
Vanadium trichlorideIdentifiers CAS number 7718-98-1 
PubChem 62647 ChemSpider 10801024 
RTECS number YW2800000 Jmol-3D images Image 1 - [V+3].[Cl-].[Cl-].[Cl-]
Properties Molecular formula VCl3 Molar mass 157.30 g/mol Appearance violet crystals
paramagneticDensity 3.0 g/cm3 (20 °C) Melting point > 300 °C (decomp)
Solubility in water soluble Structure Crystal structure Trigonal, hR24 Space group R-3, No. 148 Hazards EU Index Not listed Flash point Non-flammable Related compounds Other anions vanadium trifluoride, vanadium(III) sulfide, vanadium tribromide Other cations titanium trichloride, chromium(III) chloride, niobium trichloride, tantalum trichloride Related compounds vanadium dichloride, vanadium tetrachloride  chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Vanadium trichloride is the inorganic compound with the formula VCl3. This purple salt is a common precursor to other vanadium(III) complexes.[1]
Contents
Structure
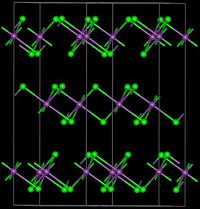
VCl3 has the common BiI3 structure, a motif that features hexagonally closest-packed chloride framework with vanadium ions occupying the octahedral holes. VBr3 and VI3 adopt the same structure, but VF3 features a structure more closely related to ReO3. VCl3 is paramagnetic and has two unpaired electrons.
Preparation and reactions
VCl3 is prepared by heating VCl4 at 160-170 °C under a flowing stream of inert gas, which sweeps out the Cl2. The bright red liquid converts to a crusty purple solid. Further heating of VCl3 decomposes with volatilization of VCl4, leaving VCl2.[2] Upon heating under H2 at 675 °C (but less than 700 °C), VCl3 reduces to greenish VCl2.
-
- 2 VCl3 + H2 → 2 VCl2 + 2 HCl
Complexes
VCl3 forms many adducts and derivatives with a broad scale of colours. VCl3 dissolves in water to give the hexahydrate, but the formula is deceptive. The salt is described by the formula [VCl2(H2O)4]Cl.2H2O. In other words, two of the water molecules are not bound to the vanadium, whose structure resembles the corresponding Fe(III) derivative. Removal of the two bound chloride ligands from [VCl2(H2O)4]+ in aqueous solution gives the green ion [V(H2O)6]3+.[3]
With tetrahydrofuran, VCl3 forms the red/pink adduct VCl3(THF)3.[4] With acetonitrile, one obtains the green adduct VCl3(MeCN)3.
When treated with KCN, VCl3 converts to [V(CN)7]4-. It is common for early metals to adopt high coordination numbers (more than 6) with compact ligands. Complementarily, larger metals can form complexes with rather bulky ligands. This aspect is illustrated by the isolation of VCl3(NMe3)2, containing two bulky NMe3 ligands.
Precursor to organometallics
The reactive species V(mesityl)3 forms from VCl3.[5]
-
- VCl3(THF)3 + 3 LiC6H2-2,4,6-Me3 → V(C6H2-2,4,6-Me3)3(THF) + 3 LiCl
This species binds CO and, under appropriate conditions, N2.
References
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Young, R. C.; Smith, M. E. "Vanadium(III) Chloride" Inorganic Syntheses volume IV, page 128-130, 1953.
- ^ Donovan, W. F.; Smith, P. W. "Crystal and Molecular Structures of Aquahalogenovanadium(1ii) Complexes. Part 1. X-Ray Crystal Structure of trans-Tetrakisaquadibromovanadium(III) Bromide Dihydrate and the lsomorphous Chloro-compound" Journal of the Chemical Society, Daltor Transactions." 1975, pages 894-896. doi:10.1039/DT9750000894
- ^ Manzer, L. E. "Tetrahydrofuran Complexes of Selected Early Transition Metals," Inorganic Synthesis. 21, 135-140, (1982).
- ^ Vivanco, M.; Ruiz, J.; Floriani, C.; Chiesi-Villa, A.; Rizzoli, C. "Chemistry of the vanadium-carbon .sigma. bond. 1. Insertion of carbon monoxide, isocyanides, carbon dioxide, and heterocumulenes into the V-C bond of Tris(mesityl)vanadium(III)" Organometallics1993 volume 12, 1794-1801. doi:10.1021/om00029a042
Vanadium compounds Categories:- Vanadium compounds
- Chlorides
- Metal halides
Wikimedia Foundation. 2010.
