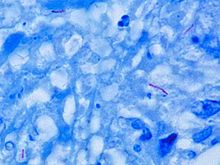
- Mycobacterium tuberculosis
-
Mycobacterium tuberculosis 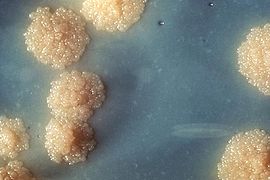
M. tuberculosis bacterial colonies Scientific classification Kingdom: Bacteria Phylum: Actinobacteria Class: Actinobacteria Order: Actinomycetales Suborder: Corynebacterineae Family: Mycobacteriaceae Genus: Mycobacterium Species: M. tuberculosis Binomial name Mycobacterium tuberculosis
Zopf 1883Synonyms Tubercle bacillus Koch 1882
Mycobacterium tuberculosis (MTB) is a pathogenic bacterial species in the genus Mycobacterium and the causative agent of most cases of tuberculosis (TB).[1] First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on its cell surface (primarily mycolic acid), which makes the cells impervious to Gram staining, so acid-fast detection techniques are used, instead. The physiology of M. tuberculosis is highly aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, MTB infects the lungs. The most frequently used diagnostic methods for TB are the tuberculin skin test, acid-fast stain, and chest radiographs.[1]
The M. tuberculosis genome was sequenced in 1998.[2][3]
Contents
Pathophysiology
M. tuberculosis requires oxygen to grow. It does not retain any bacteriological stain due to high lipid content in its wall, and thus is neither Gram-positive nor Gram-negative; hence Ziehl-Neelsen staining, or acid-fast staining, is used. While mycobacteria do not seem to fit the Gram-positive category from an empirical standpoint (i.e., they do not retain the crystal violet stain), they are classified as acid-fast Gram-positive bacteria due to their lack of an outer cell membrane.[1]
M. tuberculosis divides every 15–20 hours, which is extremely slow compared to other bacteria, which tend to have division times measured in minutes (Escherichia coli can divide roughly every 20 minutes). It is a small bacillus that can withstand weak disinfectants and can survive in a dry state for weeks. Its unusual cell wall, rich in lipids (e.g., mycolic acid), is likely responsible for this resistance and is a key virulence factor.[4]
When in the lungs, M. tuberculosis is taken up by alveolar macrophages, but they are unable to digest the bacterium. Its cell wall prevents the fusion of the phagosome with a lysosome. Specifically, M. tuberculosis blocks the bridging molecule, early endosomal autoantigen 1 (EEA1); however, this blockade does not prevent fusion of vesicles filled with nutrients. Consequently, the bacteria multiply unchecked within the macrophage. The bacteria also carried the UreC gene, which prevents acidification of the phagosome.[5] The bacteria also evade macrophage-killing by neutralizing reactive nitrogen intermediates.[citation needed]
The ability to construct M. tuberculosis mutants and test individual gene products for specific functions has significantly advanced our understanding of the pathogenesis and virulence factors of M. tuberculosis. Many secreted and exported proteins are known to be important in pathogenesis.[6]
Strain variation
M. tuberculosis comes from the genus Mycobacterium, which is composed of approximately 100 recognized and proposed species. The most familiar of the species are M. tuberculosis and M. leprae (leprosy).[7] M. tuberculosis appears to be genetically diverse, which results in significant phenotypic differences between clinical isolates. M. tuberculosis exhibits a biogeographic population structure, and different strain lineages are associated with different geographic regions. Phenotypic studies suggest this strain variation never has implications for the development of new diagnostics and vaccines. Microevolutionary variation affects the relative fitness and transmission dynamics of antibiotic-resistant strains.[8]
Hypervirulent strains
Mycobacterium outbreaks are often caused by hypervirulent strains of M. tuberculosis. In laboratory experiments, these clinical isolates elicit unusual immunopathology, and may be either hyperinflammatory or hypoinflammatory. Studies have shown the majority of hypervirulent mutants have deletions in their cell wall-modifying enzymes or regulators that respond to environmental stimuli. Studies of these mutants have indicated the mechanisms that enable M. tuberculosis to mask its full pathogenic potential, inducing a granuloma that provides a protective niche, and enable the bacilli to sustain a long-term, persistent infection.[9]
Microscopy
M. tuberculosis is characterized by caseating granulomas containing Langhans giant cells, which have a "horseshoe" pattern of nuclei. Organisms are identified by their red color on acid-fast staining.
Genome
The genome of the H37Rv strain was published in 1998.[10] Its size is 4 million base pairs, with 3959 genes; 40% of these genes have had their function characterised, with possible function postulated for another 44%. Within the genome are also six pseudogenes.
The genome contains 250 genes involved in fatty acid metabolism, with 39 of these involved in the polyketide metabolism generating the waxy coat. Such large numbers of conserved genes show the evolutionary importance of the waxy coat to pathogen survival.
About 10% of the coding capacity is taken up by two clustered gene families that encode acidic, glycine-rich proteins. These proteins have a conserved N-terminal motif, deletion of which impairs growth in macrophages and granulomas.[11]
Nine noncoding sRNAs have been characterised in M. tuberculosis,[12] with a further 56 predicted in a bioinformatics screen.[13]
Symptoms
Only an estimated 10% of people infected with M. tuberculosis ever develop the disease, and many of those have the disease only for the first few years following infection, even though the bacillus may lie dormant in the body for decades.[14]
The symptoms that patients infected with M. tuberculosis may experience are usually absent until the disease has become more complicated. It may take many months from the time the infection initially gets into the lungs until symptoms develop.[15] A cough is, however, the first symptom of the infection with M. tuberculosis.[16] The initial symptoms, including loss of appetite, fever, productive cough and loss of energy or loss of weight or night sweats, are not specific and might be easily attributed to another condition.
Primary pulmonary tuberculosis is the first stage of the condition, and it may cause fever, dry cough and some abnormalities that may be noticed on a chest X-ray. In most cases, though, primary infections tend to cause no symptoms that people do not overcome. This condition resolves itself, although it returns in more than half of the cases.
Tuberculosis-causing lung disease may result in tuberculous pleuritis, a condition that may cause symptoms such as chest pain, nonproductive cough and fever. Moreover, infection with M. tuberculosis can spread to other parts of the body, especially in patients with a weakened immune system. This condition is referred to as miliary tuberculosis, and people contacting it may experience fever, weight loss, weakness and a anorexia. In more rare cases, miliary tuberculosis can cause coughing and difficulty breathing.
Dormant (inactive) tuberculosis may return after a certain period of time, and it usually occurs in the upper lungs, causing severe symptoms, such as common cough with a progressive increase in production of mucus and coughing up blood.[14] Most patients also develop fever, loss of appetite, unexplained weight loss and night sweats.
In cases in which the infection spreads to other parts of the body, additional symptoms may occur, depending on the exact site of the spread. If the infection spreads to the abdominal cavity, symptoms such as fatigue, swelling, slight tenderness and appendicitis-like pain are likely to occur. Also, painful urination might be a sign the infection has reached the bladder. In children, M. tuberculosis infections may affect the bones, causing mild swelling and minimal pain. Fever, headache, nausea, drowsiness, and, if untreated, coma and brain damage may occur if the brain has been affected.[16] If the infection affects the pericardium, symptoms and signs such as fever, enlarged neck veins, and shortness of breath may develop. Kidney damage and the symptoms emerging with it, as well as sterility, may occur if the kidney and the reproductive system are affected, respectively.
Diagnosis
Sputum is taken on three successive mornings as the number of organisms could be low, and the specimen is treated with 3% KOH or NaOH for liquefaction and decontamination. Gram stain should never be performed, as the organism is an "acid-fast bacillus" (AFB), meaning it retains certain stains after being treated with acidic solution. In the most common staining technique, the Ziehl-Neelsen stain, AFBs are stained a bright red, which stands out clearly against a blue background; therefore, the bacteria are sometimes called "red snappers".[17] The reason for the acid-fast staining is because of its thick waxy cell wall.[18] The waxy quality of the cell wall is mainly due to the presence of mycolic acids. This waxy cell wall also is responsible for the typical caseous granuloma formation in tuberculosis. The component responsible, trehalose dimycolate, is called the cord factor. A grading system exists for interpretation of the microscopic findings based on the number of organisms observed in each field. Patients of pulmonary tuberculosis show AFBs in their sputum in only 50% of cases, which means, even if no organisms are observed, further investigation is still required. AFBs can also be visualized by fluorescent microscopy using auramine-rhodamine stain for screening, which makes them appear somewhat golden in color. Also, M. tuberculosis traditionally is grown on a selective medium, Lowenstein-Jensen medium. However, this method is quite slow, as this organism requires six to eight weeks to grow, which delays reporting of results. A faster result can now be obtained using Middlebrook medium or BACTEC.
Using BACTEC, the growth may be detected in about a week using C-14 labelled substrates. Culture media contains C-14 labelled palmitic acid. Mycobacteria metabolise these substrates and release radioactively labelled Carbon dioxide. The instrument measures labelled carbon dioxide and reports in terms of a 'growth index'. A growth index of 10 or more is considered as positive. This method can also differentiate between M. tuberculosis and M. bovis. As M. bovis is susceptible to TCH, incorporation of TCH in the medium inhibits the growth of M. bovis (i.e. the growth index decreases) but not that of M. tuberculosis.
Another rapid method for the detection of M. tuberculosis is Mycobacterial growth indicator tube (MGIT). It is a non-radiometric, automated method. It consists of tubes containing liquid culture media, and a fluorescent compound is embedded on the bottom of the tube. The fluorescent compound is sensitive to the dissolved oxygen in the liquid medium. When mycobacteria grow, they deplete the dissolved oxygen in the liquid medium and allows the compound to fluoresce brightly which can be detected by observing the tube under UV light. The results are obtained in 8 to 14 days.
During an advanced stage of tuberculosis, the organism may infect almost any part of the body, which means the specimen chosen should be appropriate for the symptoms or tissues (e.g. intestinal tuberculosis-stool).
An immunochromatographic serological assay for the diagnosis of M. tuberculosis has also been developed.[19]
Treatment
Treatment is usually administered on an outpatient basis, and consists mainly of medications. Usually, the treatment is given for six to nine months according to a therapy regimen consisting of two months of isoniazid, rifampin, and pyrazinamide, four months of isoniazid and rifampin, and ethambutol or streptomycin until the drug sensitivity is known.[20] The drug treatment schema may be changed according to the laboratory results.
Antibiotics are usually part of therapy in people who have no symptoms and whose germs are in inactive state, because they are helpful in preventing the activation of the infection. The antibiotic used is isoniazid (INH), usually taken for six to 12 months, to prevent future activation.[21] This medicine may not, however, be taken during pregnancy or in people who suffer from liver disease or alcoholism. Moreover, several side effects have been reported; some can be even life-threatening. One of the side effects caused by this drug is peripheral neuropathy, meaning a decreased sensation in the extremities and which is normally prevented or avoided by administering vitamin B6 at the same time with isoniazid.
Patients who have active bacteria are usually treated with a combination of medications; the primary antibiotic, isoniazid, is used in conjunction rifampin, ethambutol and pyrazinamide.
Streptomycin, a drug given by injection, may be used, as well, particularly when the disease is extensive and/or the patients do not take their oral medications reliably (termed "poor compliance").[21]
Usually, treatment lasts for few months, but it can even be administered for years in some cases. Mainly, the success rate of the treatment is closely related to the patient's compliance and ability to take the drugs as prescribed.
History
M. tuberculosis, then known as the "tubercle bacillus", was first described on 24 March 1882 by Robert Koch, who subsequently received the Nobel Prize in physiology or medicine for this discovery in 1905; the bacterium is also known as "Koch's bacillus".[22]
Tuberculosis has existed throughout history, but the name has changed frequently over time. In 1720, though, the history of tuberculosis started to take shape into what is known of it today; as the physician Benjamin Marten described in his A Theory of Consumption, tuberculosis may be caused by small living creatures that are transmitted through the air to other patients.[23]
See also
References
- ^ a b c Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 0-8385-8529-9.
- ^ Cole ST, Brosch R, Parkhill J, et al. (June 1998). "Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence". Nature 393 (6685): 537–44. doi:10.1038/31159. PMID 9634230.
- ^ Camus JC, Pryor MJ, Médigue C, Cole ST (October 2002). "Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv". Microbiology (Reading, Engl.) 148 (Pt 10): 2967–73. PMID 12368430. http://mic.sgmjournals.org/cgi/pmidlookup?view=long&pmid=12368430.
- ^ Murray PR, Rosenthal KS, Pfaller MA (2005). Medical Microbiology. Elsevier Mosby.
- ^ Bell E (October 2005). "Vaccines: A souped-up version of BCG". Nature Reviews Immunology 5 (10): 746. doi:10.1038/nri1720.
- ^ Wooldridge K (editor) (2009). Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis. Caister Academic Press. ISBN 978-1-904455-42-4.
- ^ (Page 576;Textbook of Diagnostic Microbiology, Mahon, Lehman, Manuselis)
- ^ Gagneux S (2009). "Strain Variation and Evolution". Mycobacterium: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-40-0.
- ^ Casali N (2009). "Hypervirulent Mycobacterium tuberculosis". Mycobacterium: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-40-0.
- ^ "Mycobacterium tuberculosis". Sanger Institute. 2007-03-29. http://www.sanger.ac.uk/Projects/M_tuberculosis/. Retrieved 2008-11-16.
- ^ Glickman MS, Jacobs WR (February 2001). "Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline". Cell 104 (4): 477–85. doi:10.1016/S0092-8674(01)00236-7. PMID 11239406. http://linkinghub.elsevier.com/retrieve/pii/S0092-8674(01)00236-7.
- ^ Arnvig KB, Young DB (August 2009). "Identification of small RNAs in Mycobacterium tuberculosis". Mol. Microbiol. 73 (3): 397–408. doi:10.1111/j.1365-2958.2009.06777.x. PMC 2764107. PMID 19555452. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0950-382X&date=2009&volume=73&issue=3&spage=397. Retrieved 2010-08-31.
- ^ Livny J, Brencic A, Lory S, Waldor MK (2006). "Identification of 17 Pseudomonas aeruginosa sRNAs and prediction of sRNA-encoding genes in 10 diverse pathogens using the bioinformatic tool sRNAPredict2". Nucleic Acids Res. 34 (12): 3484–93. doi:10.1093/nar/gkl453. PMC 1524904. PMID 16870723. http://nar.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=16870723. Retrieved 2010-08-31.[dead link]
- ^ a b "Tuberculosis Symptoms". http://www.emedicinehealth.com/tuberculosis/page3_em.htm#Tuberculosis%20Symptoms. Retrieved 2010-06-18.
- ^ "What are the symptoms of tuberculosis?". http://www.medicinenet.com/tuberculosis/page3.htm#toce. Retrieved 2010-06-18.
- ^ a b "Tuberculosis". http://www.merck.com/mmhe/sec17/ch193/ch193a.html. Retrieved 2010-06-18.
- ^ Flowers T (1995). "Quarantining the noncompliant TB patient: catching the "Red Snapper"". Journal of health and hospital law : a publication of the American Academy of Hospital Attorneys of the American Hospital Association 28 (2): 95–105. PMID 10141473.
- ^ Madigan M, Martinko J (editors) (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 0-13-144329-1.
- ^ Reddy JR, Kwang J, Lechtenberg KF, Khan NC, Prasad RB, Chengappa MM (January 2002). "An immunochromatographic serological assay for the diagnosis of Mycobacterium tuberculosis". Comp. Immunol. Microbiol. Infect. Dis. 25 (1): 21–7. doi:10.1016/S0147-9571(01)00016-9. PMID 11831744.
- ^ "Tuberculosis Treatment". http://www.emedicinehealth.com/tuberculosis/page6_em.htm#Tuberculosis%20Treatment. Retrieved 2010-06-18.
- ^ a b "How is tuberculosis treated?". http://www.medicinenet.com/tuberculosis/page5.htm#tocg. Retrieved 2010-06-18.
- ^ "Robert Koch and Tuberculosis: Koch's Famous Lecture". Nobel Foundation. 2008. http://nobelprize.org/educational_games/medicine/tuberculosis/readmore.html. Retrieved 2008-11-18.
- ^ "Tuberculosis History Timeline". http://www.mycobacteriumtuberculosis.net/history.html. Retrieved 2010-06-18.
External links
- Database of Mycobacterium tuberculosis genome sequences and related information.
Actinobacteria (high-G+C) Infectious diseases · Bacterial diseases: G+ (primarily A00–A79, 001–041, 080–109) Actinomycineae Actinomyces israelii (Actinomycosis, Cutaneous actinomycosis) · Tropheryma whipplei (Whipple's disease) · Arcanobacterium haemolyticum (Arcanobacterium haemolyticum infection)Corynebacterineae Bifidobacteriaceae Gardnerella vaginalisCategories:- Acid fast bacilli
- Mycobacteria
- Tuberculosis
- Pathogenic bacteria
Wikimedia Foundation. 2010.