- Titanocene dichloride
-
Titanocene dichloride 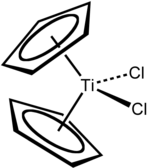
 dichloridobis (η5- cyclopentadienyl) titaniumOther namestitanocene dichloride, dichlorobis(cyclopentadienyl)titanium(IV)
dichloridobis (η5- cyclopentadienyl) titaniumOther namestitanocene dichloride, dichlorobis(cyclopentadienyl)titanium(IV)Identifiers CAS number 1271-19-8 
RTECS number XR2050000 Properties Molecular formula C10H10Cl2Ti Molar mass 248.96 g/mol Appearance bright red solid Density 1.60 g/cm3, solid Melting point 289 °C
Solubility in water sl. sol. with hydrolysis Structure Crystal structure Triclinic Coordination
geometryDist. tetrahedral Hazards R-phrases R37, R38 S-phrases S36 NFPA 704 Related compounds Related compounds Ferrocene
Zirconocene dichloride
Hafnocene dichloride
Vanadocene dichloride
Niobocene dichloride
Tantalocene dichloride
Molybdenocene dichloride
Tungstenocene dichloride
TiCl4 dichloride (verify) (what is:
dichloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air.[1] Cp2TiCl2 does not adopt the typical "sandwich" structure like ferrocene due to the 4 ligands around the metal centre, but rather takes on a distorted tetrahedral shape.[2]
Contents
Preparation
Cp2TiCl2 continues to be prepared from titanium tetrachloride, in the same way as its original synthesis by Wilkinson and Birmingham:[3]
- 2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl
The reaction is conducted in THF. Work-up sometimes washing with hydrochloric acid to convert hydrolysis derivatives to the dichloride. Recrystallization from toluene forms acicular crystals.
Cp2TiCl2 can also be prepared by using freshly distilled cyclopentadiene:- 2 C5H6 + TiCl4 → (C5H5)2TiCl2 + 2 HCl
This reaction is conducted under a nitrogen atmosphere and by using THF as solvent. The product is purified by soxhlet extraction using toluene as solvent.[4]
The complex is pseudotetrahedral. Each of the two Cp rings are attached as η5 ligands. Viewing the Cp ligands as tridentate, the complex has a coordination number of 8.
Applications in organic synthesis
Cp2TiCl2 is a generally useful reagent that effectively behaves as a source of Cp2Ti2+. A large range of nucleophiles will displace chloride. Examples:
- The Petasis reagent, Cp2Ti(CH3)2, is prepared from the action of CH3MgCl or MeLi on Cp2TiCl2. This reagent is useful for the conversion of esters into vinyl ethers.
- The Tebbe's reagent Cp2TiCl(CH2)Al(CH3)2, arises by the action of 2 equivalents Al(CH3)3 on Cp2TiCl2.
Reactions
Cp2TiCl2 undergoes anion exchange reactions, e.g. to give the pseudohalides. With NaSH and with polysulfide salts, one obtains the sulfido derivatives Cp2Ti(SH)2 and Cp2TiS5.
One Cp ligand can be removed from Cp2TiCl2 to give tetrahedral CpTiCl3. This conversion can be effected by with TiCl4 or by reaction with SOCl2.[5]
Ti(II) derivatives
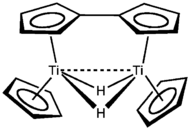
Cp2TiCl2 is a precursor to many Ti(II) derivatives. Titanocene, TiCp2, is itself so highly reactive that it is not known but it can be trapped by conducting the reduction in the presence of ligands. Reduction of titanocene dichloride results in the fulvalene complex shown in the figure.[citation needed]
Reductions have been investigated using Grignard reagent and alkyl lithium compounds. More conveniently handled reductants include Mg, Al, or Zn. The following syntheses demonstrate some of the compounds that can be generated by reduction of titanocene dichloride in the presence of π acceptor ligands.[6]
-
- Cp2TiCl2 + 2 CO + Mg → Cp2Ti(CO)2
+ MgCl2 - Cp2TiCl2 + 2 PR3 + Mg → Cp2Ti(PR3)2 + MgCl2
- Cp2TiCl2 + 2 Me3SiCCSiMe3 + Mg → Cp2TiMe3SiCCSiMe3 + MgCl2
- Cp2TiCl2 + 2 CO + Mg → Cp2Ti(CO)2
With only one equivalent of reducing agent, Ti(III) species result, i.e. Cp2TiCl.
Alkyne and benzyne derivatives of titanocene are well known.[7] One family of derivatives are the titanocyclopentadienes.[8]
Titanocene equivalents react with alkenyl alkynes followed by carbonylation and hydrolysis to form bicyclic cyclopentadienones, related to the Pauson-Khand reaction).[9] A similar reaction is the reductive cyclization of enones to form the corresponding alcohol in a stereoselective manner.[10]
Reduction of titanocene dichloride in the presence of conjugated dienes such as 1,3-butadiene gives η3-allyltitanium complexes.[11] Related reactions occur with diynes. Furthermore, titanocene can catalyze C-C bond metathesis to form asymmetric diynes.[8]
Derivatives of (C5Me5)2TiCl2
The closest relative to titanocene-ethylene complex is that derived by Na reduction of (C5Me5)2TiCl2 in the presence of ethylene. The Cp compound cannot be made. This pentamethylcyclopentadienyl (Cp*) species undergoes many reactions such as cycloadditions of alkynes.[7]
Medicinal uses
Titanocene dichloride was investigated as an anticancer drug.[12] The mechanism by which it acts is not understood, but some conjecture that it might be due to its interactions with the protein transferrin.[13]
References
- ^ S. Budaver, ed. (1989). The Merck Index (11th ed.). Merck & Co, Inc..
- ^ Clearfield, et al.; Warner, David Keith; Saldarriaga-Molina, Carlos Herman; Ropal, Ramanathan; Bernal, Ivan (1975). "Structural Studies of (π-C5H5)2 MX2 Complexes and their Derivatives. The Structure of Bis(π-cyclopentadienyl)titanium Dichloride". Canadian Journal of Chemistry 53 (11): 1621–1629. doi:10.1139/v75-228.
- ^ G. Wilkinson and J.G. Birmingham (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". Journal of the American Chemical Society 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- ^ J.M. Birmingham; Birmingham, J. M. (1964). "Synthesis of Cyclopentadienyl Metal Compounds". Adv. Organometal. Chem. 2 (17): 373. doi:10.1021/ja01646a008.
- ^ Chandra, K.; Sharma, R. K.; Kumar, N.; Garg, B. S. (1980,). "Preparation of η5-Cyclopentadienyltitanium Trichloride and η5-Methylcyclopentadienyltitanium Trichloride". Chem. Industry: 288–9.
- ^ Erik Kuester "Bis(5-2,4-cyclopentadienyl)bis(trimethylphosphine)titanium" Encyclopedia of Reagents for Organic Synthesis, 2002, John Wiley.doi:10.1002/047084289X.rn00022.
- ^ a b S.L. Buchwald and R.B. Nielsen (1988). "Group 4 Metal Complexes of Benzynes, Cycloalkynes, Acyclic Alkynes, and Alkenes". Chemical Reviews 88 (7): 1047–1058. doi:10.1021/cr00089a004.
- ^ a b U. Rosenthal, et al. (2000). "What Do Titano- and Zirconocenes Do with Diynes and Polyynes?". Chemical Reviews 33 (2): 119–129. doi:10.1021/ar9900109.
- ^ F.A. Hicks, et al. (1999). "Scope of the Intramolecular Titanocene-Catalyzed Pauson-Khand Type Reaction". Journal of the American Chemical Society 121 (25): 5881–5898. doi:10.1021/ja990682u.
- ^ N.M. Kablaoui and S.l. Buchwald (1998). "Development of a Method for the Reductive Cyclization of Enones by a Titanium Catalyst". Journal of the American Chemical Society 118 (13): 3182–3191. doi:10.1021/ja954192n.
- ^ F. Sato; Urabe, Hirokazu; Okamoto, Sentaro (2000). "Synthesis of Organotitanium Complexes from Alkenes and Alkynes and Their Synthetic Applications". Chemical Reviews 100 (8): 2835–2886. doi:10.1021/cr990277l.
- ^ R. J. Knox and P. C. McGowan. "Metallocenes as Anti-Tumour Reagents". International patent application, WO 2004/005305.
- ^ M. Guo, et al. (2000). "TiIV Uptake and Release by Human Serum Transferrin and Recognition of TiIV-Transferrin by Cancer Cells: Understanding the Mechanism of Action of the Anticancer Drug Titanocene Dichloride". Biochemistry 39 (33): 10023–10033. doi:10.1021/bi000798z. PMID 10955990.
Further reading
- Payack, J. F.; Hughes, D. L.; Cai, D.; Cottrell, I. F.; Verhoeven, T. R. "Dimethyltitanocene Titanium, bis(η5-2,4-cyclopentadien-1-yl)dimethyl-" Organic Syntheses, Coll. Vol. 10, p. 355 (2004); Vol. 79, p. 19 (2002).
- S. Gambarotta, C. Floriani, A. Chiesi-Villa and C. Guastini (1983). "Cyclopentadienyldichlorotitanium(III): a free-radical-like reagent for reducing azo (N:N) multiple bonds in azo and diazo compounds". J. Am. Chem. Soc. 105 (25): 7295–7301. doi:10.1021/ja00363a015.
- P. J. Chirik (2010). "Group 4 Transition Metal Sandwich Complexes: Still Fresh after Almost 60 Years". Organometallics 29 (7): 1500–1517. doi:10.1021/om100016p.
Categories:- Chlorides
- Metallocenes
- Organotitanium compounds
Wikimedia Foundation. 2010.