- 1,4-Dimethoxybenzene
-
1,4-Dimethoxybenzene 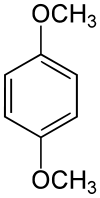 1,4-DimethoxybenzeneOther namesHydroquinone dimethyl ether; p-Methoxyanisole; 2-Benzimidazolinone; Dimethyl ether hydroquinone; USAF AN-9; Dimethylhydroquinone ether; Quinol dimethyl ether; p-Dimethoxybenzene
1,4-DimethoxybenzeneOther namesHydroquinone dimethyl ether; p-Methoxyanisole; 2-Benzimidazolinone; Dimethyl ether hydroquinone; USAF AN-9; Dimethylhydroquinone ether; Quinol dimethyl ether; p-DimethoxybenzeneIdentifiers CAS number 150-78-7 
ChemSpider 8666 
UNII 24WC6T6X0G 
RTECS number CZ6650000 Jmol-3D images Image 1 - COc1ccc(OC)cc1
Properties Molecular formula C8H10O2 Molar mass 138.16 g mol−1 Appearance White crystals Density 1.053 g/cm3[1] Melting point 53-57 °C, 326-330 K, 127-135 °F ([1])
Boiling point 213 °C, 486 K, 415 °F ([1])
Solubility in water Slightly soluble Viscosity 1.04 cP at 65 °C Structure Molecular shape Planar Hazards EU classification Irritant (Xi) R-phrases R36 R37 R38 S-phrases S26 S37 S39 Flash point 125 °C (257 °F) Autoignition
temperature795 °C (1,463 °F)[1] Related compounds  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,4-Dimethoxybenzene is the para form of dimethoxybenzene, a volatile aromatic ether with a sweet floral odor. It occurs naturally in willow (Salix) and zucchini (Cucurbita pepo). It appears to attract bees as it has a powerful response in their antenna.
Uses
Dimethoxybenzene is useful as an intermediate in synthesis of pharmaceuticals as well as other organic molecules. It is used in some paints and as a diazo dye. Cosmetically it is used on greasy skin, and with sulfur to treat acne, or as a dandruff treatment. Because of its floral odor, it is useful in perfumes and flavors. It can be used as a developer in black and white film, and as a base in synthesizing catecholamines and phenethylamines.
DMB was identified as the major psychoactive chemical in musk willow.[2]
References
- ^ a b c d 1,4-Dimethoxybenzene at Sigma-Aldrich
- ^ Isaac Karimi, Hossein Hayatgheybi, Tayebeh shamspur, Adem Kamalak, Mehrdad Pooyanmehr, Yaser Marandi (2011). "Chemical composition and effect of an essential oil of Salix aegyptiaca L. (Musk willow) in hypercholesterolemic rabbit model". Brazilian Journal of Pharmacognosy 21 (3): 407–414.
Categories:- Aromatic compounds
- Phenol ethers
Wikimedia Foundation. 2010.
