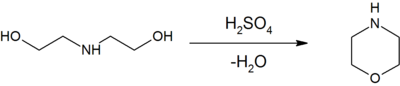- Diethanolamine
-
Diethanolamine 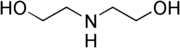 2,2'-IminodiethanolOther namesDiethanolamine, 2,2'-Iminobisethanol, Iminodiethanol, Bis(hydroxyethyl)amine, N,N-Bis(2-hydroxyethyl)amine, 2-[(2-Hydroxyethyl)amino]ethanol, 2,2'-Dihydroxydiethylamine, β,β'-Dihydroxydiethylamine, Diolamine, N-Ethylethanamine, DEA
2,2'-IminodiethanolOther namesDiethanolamine, 2,2'-Iminobisethanol, Iminodiethanol, Bis(hydroxyethyl)amine, N,N-Bis(2-hydroxyethyl)amine, 2-[(2-Hydroxyethyl)amino]ethanol, 2,2'-Dihydroxydiethylamine, β,β'-Dihydroxydiethylamine, Diolamine, N-Ethylethanamine, DEAIdentifiers CAS number 111-42-2 
PubChem 8113 ChemSpider 13835604 
UNII AZE05TDV2V 
EC number 203-868-0 KEGG D02337 
ChEBI CHEBI:28123 
ChEMBL CHEMBL119604 
RTECS number KL2975000 Jmol-3D images Image 1 - C(CO)NCCO
Properties Molecular formula C4H11NO2 Molar mass 105.14 g/mol Density 1.090 g/cm3 Melting point 28 °C, 301 K, 82 °F
Boiling point 217 °C, 490 K, 423 °F
Solubility in water Soluble Vapor pressure < 0.01 hPa (20 °C) Hazards MSDS ScienceLab.com R-phrases R20/21/22 R36/37/38 Flash point 169 °C c.c. Autoignition
temperature370 °C Explosive limits 1.7 - 6.4 %  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diethanolamine, often abbreviated as DEA, is an organic compound with the formula HN(CH2CH2OH)2. This colorless liquid is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the alcohol groups, DEA is soluble in water, and is even hygroscopic. Amides prepared from DEA are often also hydrophilic.
Contents
Production and uses
The reaction of ethylene oxide with aqueous ammonia first produces ethanolamine:
- C2H4O + NH3 → H2NCH2CH2OH
which reacts with a second and third equivalent of ethylene oxide to give DEA and triethanolamine:
- C2H4O + H2NCH2CH2OH → HN(CH2CH2OH)2
- C2H4O + HN(CH2CH2OH)2 → N(CH2CH2OH)3
About 300M kg are produced annually in this way.[1] The ratio of the products can be controlled by changing the stoichiometry of the reactants.[2]
DEA is used as a surfactant and a corrosion inhibitor. It is used to remove hydrogen sulfide and carbon dioxide from natural gas.
In oil refineries, a DEA in water solution is commonly used to remove hydrogen sulfide from various process gases. It has an advantage over a similar amine ethanolamine in that a higher concentration may be used for the same corrosion potential. This allows refiners to scrub hydrogen sulfide at a lower circulating amine rate with less overall energy usage.
DEA is a versatile chemical intermediate, principal derivatives include ethyleneimine and ethylenediamine.[1] Dehydration of DEA with sulfuric acid gives morpholine:[2]
Amides derived from DEA and fatty acids, known as diethanolamides, are amphiphilic.
Commonly used ingredients that may contain DEA
DEA is used in the production of diethanolamides, which are common ingredients in cosmetics and shampoos added to confer a creamy texture and foaming action. Consequently, some cosmetics that include diethanolamides as ingredients may contain traces of DEA.[citation needed] Some of the most commonly used diethanolamides include:
-
- Cocamide DEA
- DEA-Cetyl Phosphate
- DEA Oleth-3 Phosphate
- Lauramide DEA
- Myristamide DEA
- Oleamide DEA
- Triethanolamine
Safety
DEA is a potential skin irritant in workers sensitized by exposure to water-based metalworking fluids.[3] One study showed that DEA inhibits in baby mice the absorption of choline, which is necessary for brain development and maintenance;[4] however, a study in humans determined that dermal treatment for 1 month with a commercially available skin lotion containing DEA resulted in DEA levels that were "far below those concentrations associated with perturbed brain development in the mouse".[5] In a mouse study of chronic exposure to inhaled DEA at high concentrations (above 150 mg/m3), DEA was found to induce body and organ weight changes, clinical and histopathological changes, indicative of mild blood, liver, kidney and testicular systemic toxicity.[6] A 2009 study found that DEA has potential acute, chronic and subchronic toxicity properties for aquatic species.[7]
References
- ^ a b Matthias Frauenkron, Johann-Peter M elder, Günther Ruider, Roland Rossbacher, Hartmut Höke “Ethanolamines and Propanolamines” in Ullmann's Encyclopedia of Industrial Chemistry 2002 by Wiley-VCH, Weinheim doi:10.1002/14356007.a10_001
- ^ a b Klaus Weissermel, Hans-Jürgen Arpe, Charlet R. Lindley, Stephen Hawkins (2003). "Chap. 7. Oxidation Products of Ethylene". Industrial Organic Chemistry. Wiley-VCH. pp. 159–161. ISBN 3527305785.
- ^ Lessmann H, Uter W, Schnuch A, Geier J (2009). "Skin sensitizing properties of the ethanolamines mono-, di-, and triethanolamine. Data analysis of a multicentre surveillance network (IVDK*) and review of the literature". Contact Dermatitis 60 (5): 243–255. doi:10.1111/j.1600-0536.2009.01506.x. PMID 19397616.
- ^ Study Shows Ingredient Commonly Found In Shampoos May Inhibit Brain Development
- ^ Craciunescu, CN; Niculescu, MD; Guo, Z; Johnson, AR; Fischer, L; Zeisel, SH (2009). "Dose response effects of dermally applied diethanolamine on neurogenesis in fetal mouse hippocampus and potential exposure of humans.". Toxicological sciences : an official journal of the Society of Toxicology 107 (1): 220–6. doi:10.1093/toxsci/kfn227. PMC 2638646. PMID 18948303. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2638646.
- ^ Gamer AO, Rossbacher R, Kaufmann W, van Ravenzwaay B (2008). "The inhalation toxicity of di- and triethanolamine upon repeated exposure". Food Chem Toxicol 46 (6): 2173–83. doi:10.1016/j.fct.2008.02.020. PMID 18420328.
- ^ Libralato G, Volpi Ghirardini A, Avezzù F (2009). "Seawater ecotoxicity of monoethanolamine, diethanolamine and triethanolamine". J Hazard Mater 176 (1–3): 535–9. doi:10.1016/j.jhazmat.2009.11.062. PMID 20022426.
External links
Categories:- Alcohols
- Endocrine disruptors
- Ethylamines
Wikimedia Foundation. 2010.