- Cocamidopropyl betaine
-
Lauramidopropyl betaine 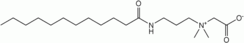
Lauramidopropyl betaine, the major component of cocamidopropyl betaine{[3-(Dodecanoylamino)propyl](dimethyl)ammonio}acetateOther names2-[(3-Dodecanamidopropyl)dimethylaminio]acetateIdentifiers CAS number 61789-40-0 
PubChem 20280 ChemSpider 19106 
EC number 263-058-8 Jmol-3D images Image 1
Image 2
Image 3- CCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC([O-])=O
CCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC(=O)[O-]
[O-]C(=O)C[N+](CCCNC(=O)CCCCCCCCCCC)(C)C
Properties Molecular formula C19H38N2O3 Molar mass 342.52 g mol−1 Exact mass 342.288243092 g/mol  betaine (verify) (what is:
betaine (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cocamidopropyl betaine (CAPB) is a synthetic surfactant derived from coconut oil and dimethylaminopropylamine.[1] It is a zwitterionic chemical compound with a quaternary ammonium cation. It is a viscous pale yellow transparent liquid and is used as a surfactant in bath products such as shampoos and hand soaps, and in cosmetics as an emulsifying agent and thickener, and to reduce irritation purely ionic surfactants would cause. It also serves as an antistatic agent in hair conditioners.
Cocamidopropyl betaine is a derivate of cocamide (a mixture of compounds derived from coconut oil) and glycine betaine (a form of betaine). See cocamide for the discussion of the length of carbon chain in the molecule.
Cocamidopropyl betaine is a medium strength surfactant which most often does not irritate skin or mucous membranes. That said, some studies indicate it is an allergen.[2][3][4] It also has antiseptic properties, making it suitable for personal sanitary products. It is compatible with other cationic, anionic, and nonionic surfactants.
Cocamidopropyl betaine to a significant degree has replaced cocamide DEA.
Contents
Specification
CAPB is obtained as an aqueous solution in concentrations of about 30%.
Typical impurities of leading manufacturers today:
- Sodium monochloroacetate < 5 ppm
- Amidoamine (AA) < 0.3%
- dimethylaminopropylamine (DMAPA) < 15 ppm
- Glycerol < 3%
However, there are qualities in the market with up to 3% AA.
The impurities AA and DMAPA are most critical, as they have been shown to be responsible for skin sensitation reactions. These by-products can be avoided by a moderate excess chloroacetate and the exact adjustment of pH value during betainization reaction accompanied by regular analytical control.
Safety
CAPB has been claimed to cause allergic reactions in some users,[2][3][4] but a controlled pilot study has found that these cases may represent irritant reactions rather than true allergic reactions.[5] Furthermore, results of human studies have shown that CAPB has a low sensitizing potential if impurities with amidoamine (AA) and dimethylaminopropylamine (DMAPA) are low and tightly controlled.[6][7] Other studies have concluded that most apparent allergic reactions to CAPB are more likely due to amidoamine.[1][8] Cocamidopropyl betaine was voted 2004 Allergen of the Year by the American Contact Dermatitis Society.[9]
See also
References
- ^ a b Foti C, Bonamonte D, Mascolo G, Corcelli A, Lobasso S, Rigano L, Angelini G. The role of 3-dimethylaminopropylamine and amidoamine in contact allergy to cocamidopropylbetaine. Contact Dermatitis. 2003 Apr;48(4):194-8. doi:10.1034/j.1600-0536.2003.00078.x PMID 12786723 full textPDF (92.0 KiB)
- ^ a b de Groot AC, van der Walle HB, Weyland JW. Contact allergy to cocamidopropyl betaine. Contact Dermatitis. 1995 Dec;33(6):419-22. PMID 8706401
- ^ a b Brand R, Delaney TA. Allergic contact dermatitis to cocamidopropylbetaine in hair shampoo. Australas J Dermatol. 1998 May;39(2):121-2. PMID 9611386
- ^ a b Mowad CM.Cocamidopropyl betaine allergy. Am J Contact Dermat. 2001 Dec;12(4):223-4. PMID 11753899
- ^ Shaffer KK, Jaimes JP, Hordinsky MK, Zielke GR, Warshaw EM. Allergenicity and cross-reactivity of coconut oil derivatives: A double-blind randomized controlled pilot study. Department of Dermatology, School of Medicine, University of Minnesota, MN, USA. Dermatitis. 2006 Jun;17(2):71-6. PMID 16956456
- ^ Fowler JF Jr, Zug KM, Taylor JS, Storrs FJ, Sherertz EA, Sasseville DA, Rietschel RL, Pratt MD, Mathias CG, Marks JG, Maibach HI, Fransway AF, Deleo VA, Belsito DV. Allergy to cocamidopropyl betaine and amidoamine in North America. Dermatitis. 2004 Mar;15(1):5-6. PMID 15573641
- ^ Korting HC, Parsch EM, Enders F, Przybilla B. Allergic contact dermatitis to cocamidopropyl betaine in shampoo. Department of Dermatology, Ludwig-Maximilians University Munich, Germany. J Am Acad Dermatol. 1992 Dec;27(6 Pt 1):1013-5. PMID 1479082
- ^ Fowler JF, Fowler LM, Hunter JE, Allergy to cocamidopropyl betaine may be due to amidoamine: a patch test and product use test study. Family & Occupational Dermatology, Inc., Louisville, Kentucky 40202, USA. Contact Dermatitis. 1997 Dec;37(6):276-81. doi:10.1111/j.1600-0536.1997.tb02464.x PMID 9455630
- ^ http://www.contactderm.org/i4a/pages/index.cfm?pageid=3467 Template:Date=September 2011
External links
Categories:- Zwitterionic surfactants
- Antiseptics
- Cosmetics chemicals
- Antistatic agents
- Quaternary ammonium compounds
- CCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC([O-])=O
Wikimedia Foundation. 2010.
