- Hafnium(IV) chloride
-
Hafnium(IV) chloride 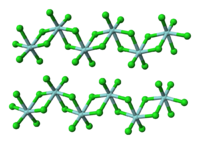 Hafnium(IV) chloride
Hafnium(IV) chloride
Hafnium tetrachlorideIdentifiers CAS number 13499-05-3 
ChemSpider 34591 
Jmol-3D images Image 1 - Cl[Hf](Cl)(Cl)Cl
Properties Molecular formula HfCl4 Molar mass 320.30 g/mol Appearance white crystalline solid Melting point 432 °C (705 K)
Solubility in water decomposes Vapor pressure 1 mmHg at 190 °C Structure Crystal structure Monoclinic Coordination
geometry4 Hazards MSDS MSDS EU Index Not listed Main hazards irritant and corrosive Flash point Non-flammable Related compounds Other anions Hafnium(IV) fluoride
Hafnium(IV) bromide
Hafnium(IV) iodideOther cations Titanium(IV) chloride
Zirconium(IV) chloride chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It functions as a Lewis acid and catalyst for certain alkylation and isomerism reactions. Hafnium the metal center in the complex occurs in nature in small amounts associated with Zirconium minerals such as zircon, cyrtolite and baddeleyite. Zircon contains 0.05 to 2.0 percent Hafnium oxide HfO2, cyrtolite with 5.5 to 17 percent HfO2 and baddeleyite contains 1.0 to 1.8 percent HfO2.[1]
Hafnium and zirconium compounds are extracted from ores together because Hafnium and Zirconium have very similar chemical and physical properties. Therefore in the synthesis of Hafnium(IV) chloride which requires HfO2, the HfO2 has to be purified from the mineral ore and this can be achieved through decomposition.[1]
Contents
Preparation
HfCl4 is produced by several procedures: (1) the reaction of carbon tetrachloride and hafnium oxide at above 450 °C;[2] (2) chlorination of a mixture of HfO2 and carbon above 600 °C;[3] and (3) chlorination of hafnium carbide above 250 °C.[4]
Hafnium(IV) chloride is prepared by heating a mixture of HfO2 and Carbon in a stream of Chlorine or Sulfur monochloride under hot temperatures as shown in the equation below.[5]
-
- HfO2 + 4Cl + C → HfCl4 + CO2
HfCl4 could also be prepared by reacting Hf(OH)4 with HCl and then boiling the reactants until crystals are formed \. Since Hf mineral ores contain Zr compounds as well, some ZrCl4 is also formed in the process of synthesizing HfCl4 therefore the product has to be purified in order to obtain pure HfCl4.
Purification
The purification of HfCl4 tends to be a difficult endeavor because Hf and Zr have very similar chemical and physical properties. Both metals have the same number of d electrons and they have a small difference in atomic radii (hafnium atomic radius is 156.4 pm while that of Zr is 160 pm).[6] This means that these two metals undergo similar reactions and form the similar coordination complexes which makes the separation of these so formed complexes difficult.
The Tetrahalides can also be separated by selectively reducing the Zirconium tetrahalide to one or more lower halides or even Zirconium. The Hafnium tetrachloride remains substantially unchanged during the reduction and may be recovered readily from the zirconium subhalides. Hafnium tetrachloride is volatile and can therefore easily be separated from the involatile zirconium trihalide. This is based on the difference in the reducibility between the two tetrahalides.[1]
There have been a number of proposed processes that could be used to purify HfCl4 from ZrCl4 and some of the proposed methods include fractional distillation, fractional precicpitation, fractional crystallization and ion exchange.[7]
Structure and bonding
This Group 4 halide contains hafnium in the +4 oxidation state. Solid HfCl4 is a polymer, each of the Hf centers are bridged by chloride ligands as found for ZrCl4. In the gas phase, both ZrCl4 and HfCl4 are expected to adopt the monomeric tetrahedral structure seen for TiCl4.[8] Electronographic investigations of HfCl4 in gas phase showed that the Hf-Cl internuclear distance is 2.33 Å and the Cl…Cl internuclear distance is 3.80 Å. The ratio of intenuclear distances r(Me-Cl)/r(Cl…Cl) is 1.630 and this value agrees well with the value for the regular tetrahedron model (1.633).[9]
Reactivity
The compound is highly reactive toward water, evolving hydrogen chloride. Aged samples often are contaminated with the oxychloride, which is also colourless. The THF complex is monomeric and thus soluble in organic solvents, which allows this hafnium complex to react more easily.[10]
-
- HfCl4 + 2 OC4H8 → HfCl4(OC4H8)2
Little is known about Hf(III) compounds because HfCl4 is especially difficult to reduce, but reduction can be effected with potassium-sodium alloy:[11]
-
- 2 HfCl4 + 2 K + 4 P(C2H5)3 → Hf2Cl6[P(C2H5)3]4 + 2 KCl
Deep green Hf2Cl6[P(C2H5)3]4 crystals form, which are diamagnetic. X-ray crystallography shows that Hf2Cl6[P(C2H5)3]4 has an edge-shared bioctahedral structure, very similar to the Zr analogue.
HfCl4 can undergo the Grignard reaction for the substitution of chloride ligands. This can be achieved by reacting HfCl4 with a Grignard reagent of general formula R-Mg-X in dry diethyl ether solvent.[12]
-
- RMg-X + HfCl4 → HfX4
Uses
Hafnium chloride is the precursor to highly active catalysts for the polymerization of alkenes, especially propylene.[13] Typical catalysts are derived from tetrabenzylhafnium.
HfCl4 is an effective Lewis acid for various applications in organic synthesis. For example, ferrocene is alkylated with allyldimethylchlorosilane more efficiently using hafnium chloride relative to aluminium trichloride. The greater size of Hf may diminish HfCl4's tendency to complex to ferrocene.[14]
HfCl4 increases the rate and control of 1,3-dipolar cycloadditions.[15] It was found to yield better results than other Lewis acids when used with aryl and aliphatic aldoximes, allowing specific exo-isomer formation.
Hafnium chloride is also the precursor to highly active catalysts for the polymerization of alkenes, especially propylene. Typical catalysts are derived from tetrabenzylhafnium. It was recently reported that MgCl2 supported HfCl4 in combination with AlEt3 or Dimethyltitanocene specifically produces polypropylene.[16]
Materials science applications
HfCl4 is the most common precursor for chemical vapor deposition of hafnium dioxide and hafnium silicate, used as high-k dielectrics in manufacture of modern high-density integrated circuits.
References
- ^ a b c Ivan Edgar Newnham. Purification of Hafnium Tetrachloride. U.S. patent 2,961,293 November 22, 1960.
- ^ Encyclopedia of Chemical Technology. Kirk-Othermer. Vol.11, 4th Ed. (1991)
- ^ Hala, J. Halides, Oxyhalides and Salts of Halogen Complexes of Titanium, Zirconium, Hafnium, Vanadium, Niobium and Tantalum. Vol. 40, 176-177, (1970).
- ^ S.V. Elinson, K.I. Petrov. Analytical Chemistry of the Elements: Zirconium and Hafnium. 11, (1969).
- ^ B.S Hopkins. Chapters in the chemistry of less familiar elements, Chapter 13 Hafnium; pp 7
- ^ V. P. Spiridonov, P. A. Akishin and V. I. Tsirel’nikov, M. V. Lomonosov. Electronographic Investigation of the structure of Zirconium and Hafnium Tetrachloride Molecules in Gas Phase. Journal of Structural Chemistry [Online] Vol. 3, 1962, pp 329-330
- ^ Wikipedia contributors. Wikipedia Free Encyclopedia. Hafnium (IV) Chloride, December 2009, pg version ID 331590363.
- ^ Greenwood, N. N., Earnshaw, A. Chemistry of the Elements Second Ed. Butterworth-Heinemann, Boston, (1997).
- ^ V. P. Spiridonov, P. A. Akishin and V. I. Tsirel’nikov, M. V. Lomonosov. Electronographic Investigation of the structure of Zirconium and Hafnium Tetrachloride Molecules in Gas Phase. Journal of Structural Chemistry [Online] Vol. 3, 1962, pp 329-330.
- ^ Manxzer, L. E.; Deaton, Joe; Sharp, Paul; Schrock, R. R. (1982). "Tetrahydrofuran Complexes of Selected Early Transition Metals". Inorg. Synth.. Inorganic Syntheses 21: 135. doi:10.1002/9780470132524.ch31. ISBN 9780470132524.
- ^ M. E. Riehl, S. R. Wilson, and G. S. Girolami (1993). "Synthesis, X-ray Crystal Structure, and Phosphine-Exchange Reactions of the Hafnium(III)-Hafnium(II1) Dimer Hf2Cl6[P(C2H5)3]4". Inorg. Chem. 32 (2): 218–222. doi:10.1021/ic00054a017.
- ^ Ian Westmoreland. Alkylation of hafnium tetrachloride with benzyl Grignard; Hafnium tetrabenzyl ; Group 4 tetrabenzyl, pp 211, 2003
- ^ Ron Dagani (2003-04-07). "Combinatorial Materials: Finding Catalysts Faster". Chemical and Engineering News. pp. 10. http://pubs.acs.org/cen/topstory/8114/print/8114notw5.html.
- ^ S. Ahn, Y. Song, B. Yoo, I. Jung (2000). "Lewis Acid-Catalyzed Friedel-Crafts Alkylation of Ferrocene with Allylchlorosilanes". Organometallics 19 (14): 2777–2780. doi:10.1021/om0000865.
- ^ P. Dunn, A. Graham, R. Grigg, P. Higginson (2000). "Tandem 1,3-azaprotiocyclotransfer–cycloaddition reactions between aldoximes and divinyl ketone. Remarkable rate enhancement and control of cycloaddition regiochemistry by hafnium(iv) chloride". Chem. Commun. (20): 2035–2036. doi:10.1039/b005389i.
- ^ Francesco Masi, Francesco Menconi, Stefano Malquori, Renzo Invernizzi. Supported Hafnium Catalysts for Sterospecific polymerization of α-olefins, Dipartimento di Chimica e Chimica Industriale, Universita di Pisa, Pisa, Italy, 1989
Further reading
- Duraj, S. A.; Towns; Baker; Schupp, J. (1990). "Structure of cis-Tetrachlorobis(tetrahydrofuran)hafnium(IV)". Acta Crystallographica C46 (5): 890–2. doi:10.1107/S010827018901382X.
External links
Hafnium compounds Categories:- Chlorides
- Hafnium compounds
- Metal halides
Wikimedia Foundation. 2010.
