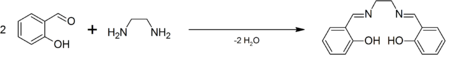- Salen ligand
-
Salen ligand 
 Other names2,2'-Ethylenebis(nitrilomethylidene)diphenol, N,N'-Ethylenebis(salicylimine)
Other names2,2'-Ethylenebis(nitrilomethylidene)diphenol, N,N'-Ethylenebis(salicylimine)Identifiers CAS number 94-93-9 
Properties Molecular formula C16H16N2O2 Molar mass 268.31 Appearance yellow solid Melting point 125-129 °C, 269 K, -75 °F
Solubility in water organic solvents  ligand (verify) (what is:
ligand (verify) (what is:  /
/ ?)
?)
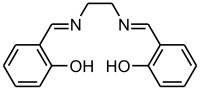
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Salen is the abbreviation for a popular chelating ligand used in coordination chemistry and homogeneous catalysis. The name salen is a contraction for salicylaldehyde and ethylenediamine. The ligand is a bright yellow micaceous solid that is soluble in polar organic solvents.
Nomenclature
The diphenol H2salen is the conjugate acid of the ligand that logically is salen2-. But the terminology is used loosely. As an anionic tetradendate ligand, salen2- resembles tetradentate ligands including those that are macrocyclic, such as porphyrinate, corrin, bis(dimethylglyoximate), and some Schiff bases.
Preparation
SalenH2 is commercially available. It was first prepared by Pfeiffer.[1] It is often generated in situ followed by the addition of the metal salt, but the ligand is also easily prepared as a pure organic compound by the condensation of ethylenediamine and salicylaldehyde.[2]
References
- ^ P. Pfeiffer, E. Breith, E. Lübbe, T. Tsumaki (1933). ""Tricyclische orthokondensierte Nebenvalenzringe". Justus Liebig's Annalen der Chemie 503: 84–130. doi:10.1002/jlac.19335030106.
- ^ Harvey Diehl, Clifford C. Hach (1950). "Bis(N,N' - Disalicylalethylenediamine) -μ - Aquodicobalt(II)". Inorg. Synth.. Inorganic Syntheses 3: 196–201. doi:10.1002/9780470132340.ch53. ISBN 9780470132340.
Categories:- Organometallic chemistry
- Imines
Wikimedia Foundation. 2010.