- Morphine total synthesis
-
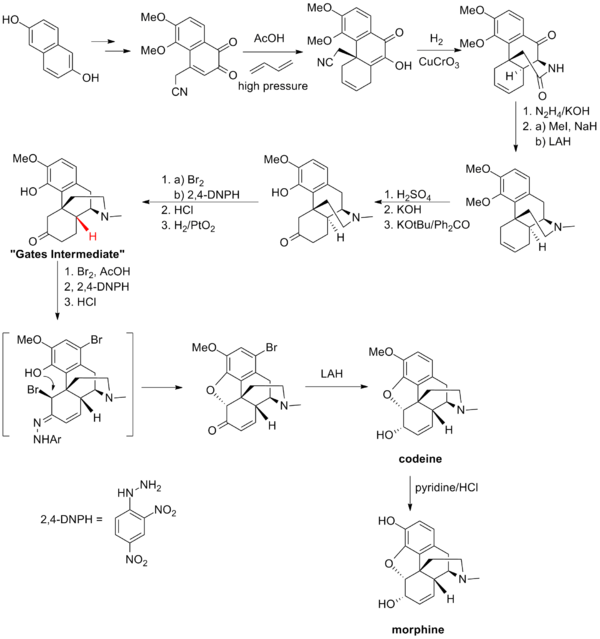
Morphine total synthesis in chemistry describes the total synthesis of the alkaloid morphine. The first synthesis by Marshall D. Gates, Jr. in 1952 is considered a classic in the field.[1][2]
Several other syntheses were reported, notably by the research groups of Rice,[3] Evans,[4] Fuchs,[5] Parker,[6] Overman,[7] Mulzer-Trauner,[8] White,[9] Taber,[10] Trost,[11] Fukuyama,[12] Guillou[13] and Stork.[14]
Gates Synthesis
Gates' total synthesis of morphine is one of the first example of the Diels-Alder reaction in the context of total synthesis.
External links
References
- ^ Gates, M. D.; Tschudi, G. J. Am. Chem. Soc. 1952, 74 (4), 1109–1110. doi:10.1021/ja01588a033
- ^ Gates, M. D.; Tschudi, G. J. Am. Chem. Soc. 1956, 78 (7), 1380–1393. doi:10.1021/ja01588a033
- ^ Rice, K. C. J. Org. Chem. 1980, 45 (15), 3135-3137. doi:10.1021/jo01303a045
- ^ Evans, D. A.; Mitch, C. H. Tetrahedron Lett., 1982, 23 (3), 285-288. doi:10.1016/S0040-4039(00)86810-0
- ^ Toth, J. E.; Hamann, P. R.; Fuchs, P. L. J. Org. Chem. 1988, 53 (20), 4694-4708. doi:10.1021/jo00255a008
- ^ Parker, K. A.; Fokas, D. J. Am. Chem. Soc., 1992, 114 (24), 9688-9689. doi:10.1021/ja00050a075
- ^ Hong, C. Y.; Kado, N.; Overman, L. E. J. Am. Chem. Soc., 1993, 115 (23), 11028-11029. doi:10.1021/ja00076a086
- ^ Mulzer, J.; Dürner, G.; Trauner, D. Angew. Chem. Int. Ed., 1996, 35 (23-24), 2830-2832. doi:10.1002/anie.199628301
- ^ White, J. D.; Hrnciar, P.; Stappenbeck, F. J. Org. Chem. 1999, 64 (21), 7871-7884. doi:10.1021/jo990905z
- ^ Taber, D. F.; Neubert, T. D.; Rheingold, A. L. J. Am. Chem. Soc., 2002, 124 (42), 12416–12417. doi:10.1021/ja027882h
- ^ Trost, B. M.; Tang, W. J. Am. Chem. Soc., 2002, 124 (49), 14542-14543. doi:10.1021/ja0283394
- ^ Uchida, K.; Yokoshima, S.; Kan, T.; Fukuyama, T. Org. Lett., 2006, 8 (23), 5311-5313. doi:10.1021/ol062112m
- ^ Varin, M.; Barré, E.; Iorga, B.; Guillou, C. Chem. Eur. J., 2008, 14 (22), 6606-6608. doi:10.1002/chem.200800744
- ^ Stork, G.; Yamashita, A.; Adams, J.; Schulte, G. R.; Chesworth, R.; Miyazaki, Y.; Farmer, J. J. J. Am. Chem. Soc., 2009, 131 (32), 11402-11406. doi:10.1021/ja9038505
Categories:- Natural opium alkaloids
- Total synthesis
Wikimedia Foundation. 2010.