- Merrilactone A
-
Merrilactone A 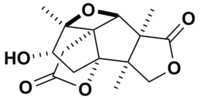 Other names(+)-Merrilactone A
Other names(+)-Merrilactone AIdentifiers CAS number 301842-37-5 Properties Molecular formula C15H18O6 Molar mass 294.3 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Merrilactone A is one of the four sesquiterpenes that were newly discovered from the fruit of Illicium merrillianum in 2000[1]. Members of the genus Illicium include Chinese star anise, widely used as a spice for flavouring food and beverages, and also poisonous plants such as Japanese star anise[2]. Chemical studies of Illicium have developed rapidly over the last 20 years, and merrilactone A has been shown to have neurotrophic activity in fetal rat cortical neuron cultures.[3] This has led researchers to believe that Merrilactone A may hold therapeutic potential in the treatment of neuro-degenerative diseases such as Alzheimer’s disease and Parkinson's disease.[4]
Contents
Occurrence
Merrilactone A occurs naturally in Illicium merrillianum, a plant indigenous to southern China and Myanmar[3]. The genus Illicium belongs to the family Illiciaceae and is an evergreen shrub or tree. Approximately 40 species are disjunctively distributed in eastern North America, Mexico, the West Indies, and eastern Asia. The highest concentration of species is in northern Myanmar and southern China, where nearly 35 species have been described. The fruits of the Illicium species are distinctive star-shaped follicles that have a characteristic refreshing flavour. The fruits of Illicium merrillianum also have an aromatic odor, bland taste and cause numbness of the tongue when chewed.[2]
The only economically important product from this genus is the fruit of Illicium verum, or Chinese star anise, which is widely used as a spice for flavouring food and beverages. In contrast, the fruit of Japanese star anise, Illicium anisatum, have been known to be very toxic for several centuries.
Biological activity
Merrilactone A was found to exhibit a significant neurotrophic activity, such as greatly promoting neurite outgrowth in the primary cultures of fetal rat cortical neurons at concentrations from 10 to 0.1 μmol/L. It was also found that this compound had a property of neuroprotection at same concentration.[1]
Biosynthesis
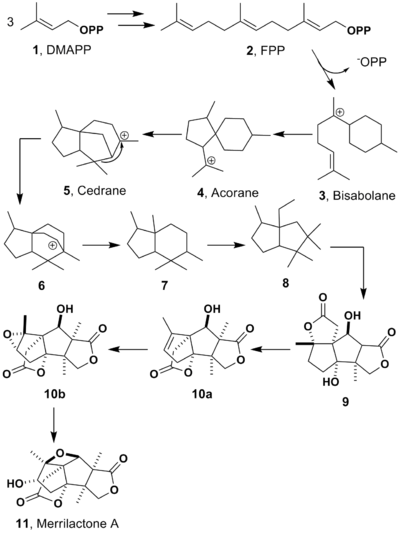
This compound is originated from mevalonic acid pathway which produce dimethylallyl diphosphate (DMAPP) from acetyl-CoA. Three DMAPPs, or one DMAPP and two isopentenyl diphosphate (IPP), are made into a farnesyl diphosphate (FPP), which is the fundamental precursor of sesquiterpene, and then sesquiterpene cyclise enzymes cause it a cyclization. The synthesis up to here is well known, though process after the first cyclization was unclear. Anislactone-type sesquiterpenes which merrilactone A belongs to has been thought to be biosynthezised from majucin since they have γ-lactone.[5] However, this pathway has trouble to elucidate some configurations and other feature they have. The biosynthesis shown in the figure was newly proposed and solved these problems. The point of this pathway is that seco-prezizaane, anislactone and tashironin group which are all found in Illicium are all derived from the same intermediate 6. This is expected to be able to give all characteristic sequiterpenes in Illicium plants a reasonable explanation of biosynthesis.
Total synthesis
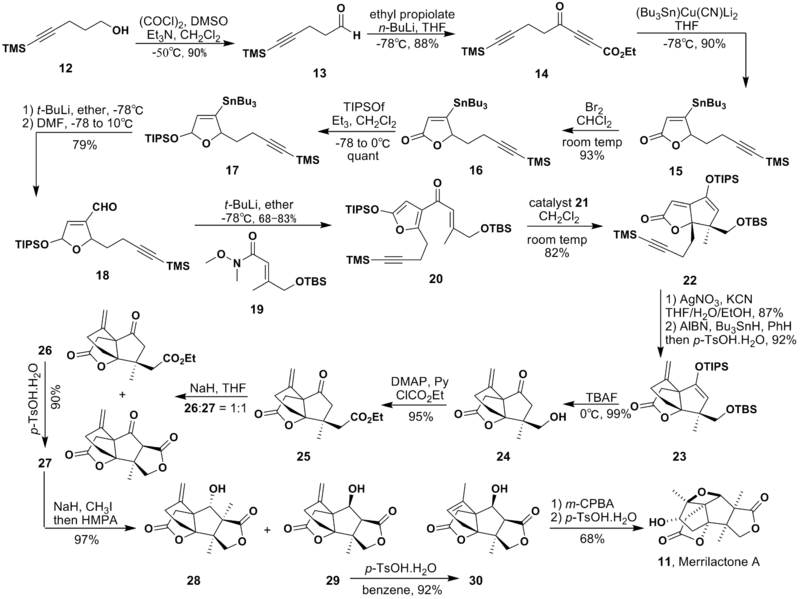
Since the discovery of merrilactone A, many methods of total synthesis have been proposed, of which four produce racemic body,[6][7][8][9] and two produce natural enantiomer.[10][11] The following part will introduce the latest one.[9]
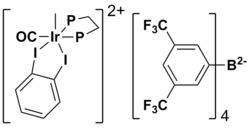
The synthesis of the ring on the back side starting from the alcohol 5 is relatively easy to accomplish and high yield. Since the strategy of this synthesis is using a Lewis acid to perform Nazarov cyclization, the next target is to make the substrate, which is 20 here. The distinguishing point is that this synthesis uses the Lewis acid-catalyzed Nazarov cyclization (20 to 22 in the figure). The Nazarov cyclization is a 4π electrocyclization typically involving the conversion of divinyl ketones to cyclopentenones by activation with a Lewis acid.[12] The Lewis acid catalyst 21 used here is shown below.
This synthesis has least number of step and generally gives high yield at every process, though the total yield could not be found.
References
- ^ a b Fukuyama, Yoshiyasu; Huang, Jian-mei; Nakade, Kousuke; Yokoyama, Ritsuko (2001-09-01). "72 (P-29) シキミ科植物(Illicium merrillianum)のアニスラクトン型セスキテルペンの構造と神経突起伸展活性(ポスター発表の部)" (in Japanese). [72 (P-29) Structure and Neurotrophic Activity of Anislactone-type Sesquiterpenes from Illicum merrillianum]. 43. Symposium on the chemistry of natural products. pp. 425–430. http://ci.nii.ac.jp/naid/110006682117. Retrieved 2009-12-16.
- ^ a b Jodral, M. M. (2004). Illicium, Pimpinella and Foeniculum. Granada: CRC Press. ISBN 0415322464. http://books.google.com/?id=uHzstuXrZi4C.
- ^ a b Huang, Jian-mei; Yokoyama, Ritsuko; Yang, Chun-shu; Yoshiyasu, Fukuyama (August 2000). "Merrilactone A, a novel neurotrophic sesquiterpene dilactone from Illicium merrillianum". Tetrahedron Letters 41 (32): 6111–6114. doi:10.1016/S0040-4039(00)01023-6.
- ^ Inoue, Masayuki; Lee, Nayoung; Kasuya, Satoshi; Sato, Takaaki; Hirama, Masahiro; Moriyama, Miyako; Fukuyama, Yoshiyasu (2007-04-13). "Total synthesis and bioactivity of an unnatural enantiomer of Merrilactone A: development of an enantioselective desymmetrization strategy". Journal of Organic Chemistry 72 (8): 3065–3075. doi:10.1021/jo0700474. PMID 17355151.
- ^ Kouno, I.; Mori, K.; Okamoto, S.; Sato, S. Structures of Anislactone A and B; Novel Type of Sesquiterpene Lactones from the Pericarps of Illicium anisatum. Chem. Pharm. Bull. 1990, 38, 3060-3063
- ^ Birman, Vladimir B.; Danishefsky, Samuel J. (2002). "The Total Synthesis of (±)-Merrilactone A". Journal of the American Chemical Society 124 (10): 2080–1. doi:10.1021/ja012495d. PMID 11878938.
- ^ Inoue, Masayuki et al. (2003). "Total Synthesis of Merrilactone A". J. Am. Chem. Soc. 125 (36): 10772–3. doi:10.1021/ja036587. PMID 12952441.
- ^ Mehta, Goverdhan; Singh, S. Robindro (2006). "Total Synthesis of (±)-Merrilactone A". Angewandte Chemie International Edition 45 (6): 953–5. doi:10.1002/anie.200503618. PMID 16381043.
- ^ a b He, Wei; Huang, Jie; Sun, Xiufeng; Frontier, Alison J. (2008). "Total Synthesis of (±)-Merrilactone A". Journal of the American Chemical Society 130 (1): 300–8. doi:10.1021/ja0761986. PMID 18067294.
- ^ Waters, Stephen P.; Tian, Yuan; Li, Yue-Ming; Danishefsky, Samuel J. (2005). "Total Synthesis of (−)-Scabronine G, an Inducer of Neurotrophic Factor Production". Journal of the American Chemical Society 127 (39): 13514–5. doi:10.1021/ja055220x. PMID 16190712.
- ^ Inoue, Masayuki; Sato, Takaaki; Hirama, Masahiro (2006). "Asymmetric Total Synthesis of (−)-Merrilactone A: Use of a Bulky Protecting Group as Long-Range Stereocontrolling Element". Angewandte Chemie International Edition 45 (29): 4843–8. doi:10.1002/anie.200601358. PMID 16795104.
- ^ Frontier, Alison J.; Collison, Christina (2005). "The Nazarov cyclization in organic synthesis. Recent advances". Tetrahedron 61 (32): 7577. doi:10.1016/j.tet.2005.05.019.
Categories:- Sesquiterpene lactones
- Total synthesis
Wikimedia Foundation. 2010.