- Mean inter-particle distance
-
Mean inter-particle distance (or mean inter-particle separation) is the mean distance between microscopic particles (usually atoms or molecules) in a macroscopic body.
Contents
Ambiguity
From the very general considerations, the mean inter-particle distance is proportional to the size of the per-particle volume 1 / n, i.e.,
where n = N / V is the particle density. However, barring a few simple cases such as the ideal gas model, precise calculations of the proportionality factor are impossible analytically. Therefore, approximate expressions are often used. One such an estimation is the Wigner-Seitz radius
which corresponds to the radius of a sphere having per-particle volume 1 / n. Another popular definition is
- 1 / n1 / 3,
corresponding to the length of the edge of the cube with the per-particle volume 1 / n. Evidently, the two definitions differ by a factor of
 , thus one has to exercise care if an article fails to define the parameter exactly. On the other hand, it is often used in qualitative statements where such a numeric factor is either irrelevant or plays an insignificant role, e.g.,
, thus one has to exercise care if an article fails to define the parameter exactly. On the other hand, it is often used in qualitative statements where such a numeric factor is either irrelevant or plays an insignificant role, e.g.,- "a potential energy ... is proportional to some power n of the inter-particle distance r" (Virial theorem)
- "the inter-particle distance is much larger than the thermal de Broglie wavelength" (Kinetic theory)
Ideal gas
Nearest neighbor distribution
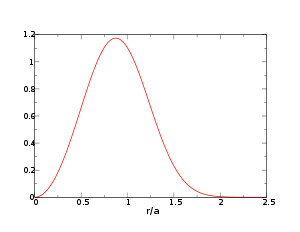
We want to calculate probability distribution function of distance to the nearest neighbor (NN) particle. (The problem was first considered by Paul Hertz;[1] for a modern derivation see, e.g.,.[2]) Let us assume N particles inside a sphere having volume V, so that n = N / V. Note that since the particles in the ideal gas are non-interacting, the probability to find a particle at a certain distance from another particle is the same as probability to find a particle at the same distance from any other point; we shall use the center of the sphere.
An NN particle at distance r means exactly one of the N particles resides at that distance while the rest N − 1 particles are at larger distances, i.e., they are somewhere outside the sphere with radius r.
The probability to find a particle at the distance from the origin between r and r + dr is
 , while the probability to find a particle outside that sphere is
, while the probability to find a particle outside that sphere is  . The sought-for expression is then
. The sought-for expression is thenwhere we substituted
Finally, taking the
 limit and using
limit and using  , we obtain
, we obtainOne can immediately check that
The distribution peaks at

Mean distance and higher NN distribution moments
or, using the t = x3 substitution,
where Γ is the gamma function. Thus,
In particular,
References
- ^ Hertz, Paul (1909). "Über den gegenseitigen durchschnittlichen Abstand von Punkten, die mit bekannter mittlerer Dichte im Raume angeordnet sind". Mathematische Annalen 67 (3): 387–398. doi:10.1007/BF01450410. ISSN 0025-5831. http://www.springerlink.com/content/q133104qq7596l37/. Retrieved 2011-03-03.
- ^ Chandrasekhar, S. (1943-01-01). "Stochastic Problems in Physics and Astronomy". Reviews of Modern Physics 15 (1): 1. Bibcode 1943RvMP...15....1C. doi:10.1103/RevModPhys.15.1. http://link.aps.org/doi/10.1103/RevModPhys.15.1. Retrieved 2011-03-01.
See also
Categories:
Wikimedia Foundation. 2010.