- Mapracorat
-
Mapracorat 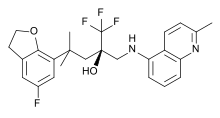
Systematic (IUPAC) name (2R)-1,1,1-trifluoro-4-(5-fluoro-2,3-dihydro-1-benzofuran-7-yl)-4-methyl-2-[[(2-methylquinolin-5-yl)amino]methyl]pentan-2-ol Clinical data Pregnancy cat. ? Legal status Investigational Routes Topical (ointment, eye drops) Identifiers CAS number 887375-26-0 
ATC code None PubChem CID 24795088 ChemSpider 25104194 
UNII 145V79YBVP 
Chemical data Formula C25H26F4N2O2 Mol. mass 462.479 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mapracorat (INN, code names BOL-303242-X, ZK 245186[1]) is an anti-inflammatory drug belonging to the experimental class of selective glucocorticoid receptor agonists (SEGRAs). It is in clinical trials for the topical treatment of atopic dermatitis[2], inflammation following cataract surgery[3], and allergic conjunctivitis[4]. Preliminary investigation for the treatment of keratoconjunctivitis sicca has been conducted in cellular models.[1]
Clinical trials
Phase II clinical trials with mapracorat started in summer 2009. One trial was a double blind dose finding study for an ointment against atopic dermatitis. It tested concentrations of 0.01%, 0.03% and 0.1% versus placebo over four weeks in around 64 patients. This trial was conducted by Intendis, a part of Bayer HealthCare Pharmaceuticals specialized on dermatology, and completed in September or October 2010.[2] The other trial, also with a double blind design, evaluated an ophthalmic suspension for the treatment of inflammation following cataract surgery. Various concentrations and dosing schemes were tested versus placebo in about 550 patients. The study was conducted by Bausch & Lomb and completed in September 2010.[3] Its successor study, a phase III trial, started in November 2010 and is scheduled to run until February 2012.[5]
As of March 2011[update] no study results are available.
References
- ^ a b Cavet, M. E.; Harrington, K. L.; Ward, K. W.; Zhang, J. Z. (2010). "Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells". Molecular vision 16: 1791–1800. PMC 2932489. PMID 20824100. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2932489.
- ^ a b ClinicalTrials.gov NCT00944632 Dose Escalation of Different Concentrations of ZK 245186 in Atopic Dermatitis
- ^ a b ClinicalTrials.gov NCT00905450 Evaluation of BOL-303242-X Versus Vehicle for the Treatment of Inflammation Following Cataract Surgery
- ^ ClinicalTrials.gov Ophthalmic Formulation in Subjects With Allergic Conjunctivitis NCT01289431Mapracorat Ophthalmic Formulation in Subjects With Allergic Conjunctivitis
- ^ ClinicalTrials.gov NCT01230125 Mapracorat Ophthalmic Suspension for the Treatment of Ocular Inflammation Following Cataract Surgery
This drug article relating to the musculoskeletal system is a stub. You can help Wikipedia by expanding it. This antineoplastic or immunomodulatory drug article is a stub. You can help Wikipedia by expanding it. This dermatologic drug article is a stub. You can help Wikipedia by expanding it.
