- Dihydroimidazol-2-ylidene
-
Dihydroimidazol-2-ylidene  Dihydroimidazol-2-ylidene[citation needed]Systematic nameImidazolidin-2-ylidene[citation needed]
Dihydroimidazol-2-ylidene[citation needed]Systematic nameImidazolidin-2-ylidene[citation needed]Identifiers ChemSpider 11350507 
Jmol-3D images Image 1 - [C]1NCCN1
Properties Molecular formula C3N2H6 Molar mass 70.0931 g mol-1 Exact mass 70.053098202 g mol-1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dihydroimidazol-2-ylidene is a hypothetical organic compound with formula C3H6N2. It would be a heterocyclic compound, formally derived from imidazolidine with two hydrogen atoms removed from carbon number 2, leaving two vacant chemical bonds — which makes it a carbene.
Although carbenes in general are extremely short-lived, some derivatives of this compound are surprisingly stable, and form an important class of the persistent carbenes. They include the first stable carbenes postulated (but not isolated) by Hans-Werner Wanzlick around 1960.[1][2][3]
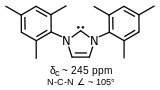 1,3-Dimesityl-imidazol-4,5-dihydro-2-ylidene, a stable carbene without delocalization around the ring.
1,3-Dimesityl-imidazol-4,5-dihydro-2-ylidene, a stable carbene without delocalization around the ring.
(external viewer)They also include an example of the (saturated) imidazolin-2-ylidene (carbene) reported by A.J. Arduengo in 1995.[4]
References
- ^ H.-W. Wanzlick and E. Schikora (1960). "Ein neuer Zugang zur Carben-Chemie". Angewandte Chemie 72 (14): 494. doi:10.1002/ange.19600721409.
- ^ H.-W. Wanzlick and E. Schikora (1960). "Ein nucleophiles Carben". Chemische Berichte 94 (9): 2389–2393. doi:10.1002/cber.19610940905.
- ^ H.-W. Wanzlick (1962). "Aspects of Nucleophilic Carbene Chemistry". Angew. Chem., Int. Ed. Engl. 1 (2): 75. doi:10.1002/anie.196200751.
- ^ A. J. Arduengo, III, H. V. R. Dias, R. L. Harlow, and M. Kline (1992). "Electronic stabilization of nucleophilic carbenes". J. Am. Chem. Soc. 114 (14): 5530. doi:10.1021/ja00040a007.
This article about a heterocyclic compound is a stub. You can help Wikipedia by expanding it.

